Article Text
Abstract
Objective We hypothesise that low 25-hydroxyvitamin D (25(OH)D) levels before hospitalisation are associated with increased risk of acute respiratory failure.
Design Retrospective cohort study.
Setting Medical and Surgical Intensive care units of two Boston teaching hospitals.
Patients 1985 critically ill adults admitted between 1998 and 2011.
Interventions None.
Measurements and main results The exposure of interest was prehospital serum 25(OH)D categorised as ≤10 ng/mL, 11–19.9 ng/mL, 20–29.9 ng/mL and ≥30 ng/mL. The primary outcome was acute respiratory failure excluding congestive heart failure determined by International Classification of Diseases Ninth Edition (ICD-9) coding and validated against the Berlin Definition of acute respiratory sistress syndrome. Association between 25(OH)D and acute respiratory failure was assessed using logistic regression, while adjusting for age, race, sex, Deyo-Charlson Index and patient type (medical vs surgical).
In the cohort, the mean age was 63 years, 45% were male and 80% were white; 25(OH)D was ≤10 ng/mL in 8% of patients, 11–19.9 ng/mL in 24%, 20–29.9 ng/mL in 24% and ≥30 ng/mL in 44% of patients. Eighteen per cent (n=351) were diagnosed with acute respiratory failure. Compared to patients with 25(OH)D ≥30 ng/mL, patients with lower 25(OH)D levels had significantly higher adjusted odds of acute respiratory failure (≤10 ng/mL, OR=1.84 (95% CI 1.22 to 2.77); 11–19.9 ng/mL, OR=1.60 (95% CI 1.19 to 2.15); 20–29.9 ng/mL, OR=1.37 (95% CI 1.01 to 1.86)).
Conclusions Prehospital 25(OH)D was associated with the risk of acute respiratory failure in our critically ill patient cohort.
- ARDS
- Clinical Epidemiology
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/
Statistics from Altmetric.com
Key messages
Vitamin D deficiency prior to hospitalization is associated with development of acute respiratory failure.
Vitamin D deficient patients who develop acute respiratory failure have heightened mortality.
Introduction
In the USA, every year, 190 600 patients develop acute respiratory distress syndrome (ARDS), with 74 500 associated deaths and 2.2 million days in the intensive care unit (ICU).1 Incidences of ARDS among patients with acute illness and mechanically ventilated patients are 7% and 11–23%, respectively.2 Although critical care outcomes in general and those associated with acute respiratory failure have improved over time, long-term outcomes of ICU survivors has gained prominence.3 ,4 Among the more than 100 000 patients who survive ARDS each year, many develop cognitive abnormalities, depression, post-traumatic stress disorders and have poor health-related quality of life.1 ,5
Recent evidence suggests that vitamin D is a key regulator of the innate and adaptive immune system.5 Serum 25-hydroxyvitamin D (25(OH)D) is the major circulating metabolite of vitamin D, the standard measure of vitamin D status and is used to assess therapeutic response to supplementation. Low serum 25(OH)D levels are associated with increased risk of viral and bacterial infections as well as sepsis possibly due to effects on innate and adaptive immunity.6 ,7 Additionally, vitamin D modulates inflammation, fibrosis and airway destruction in the lung which are major steps in ARDS pathogenesis.8 ,9 Further, decreased muscle strength and mass is associated with vitamin D deficiency.10 Recent data from a randomised controlled trial on high-dose vitamin D in critically ill patients (VITdAL-ICU trial) shows as a secondary outcome the improved survival in patients with severe vitamin D deficiency (25(OH)D≤12 ng/mL) who receive vitamin D.11 Data are lacking with regards to the association of prehospitalisation vitamin D inadequacy and incident ARDS during critical illness.
Given that vitamin D inadequacy, defined by measuring circulating 25(OH) D3 levels, is increasingly prevalent in the general population of the USA and associated with critical care outcomes, we performed a two-centre observational study of adult patients among whom 25(OH)D concentrations had been measured for routine clinical reasons within 1 year before hospitalisation.7 ,12–23 The objective of this study was to test the hypothesis that vitamin D status before hospital admission is inversely associated with the risk of developing acute respiratory failure in a critically-ill patient cohort.
Materials and methods
Data on all patients admitted to two teaching hospitals in Boston between 4 August 1998 and 12 January 2011 were obtained through a computerised registry. During the study period, there were 79 927 patient ICU admissions to the hospitals under study. Approval for the study was granted by the Partners Human Research Committee (Institutional Review Board). Requirement for consent was waived as the data were analysed anonymously. The cohort included patients ≥18 years of age who received critical care and had serum 25(OH)D measured between 7 and 365 days before hospitalisation. We excluded patients with congestive heart failure (International Classification of Diseases Ninth Edition (ICD-9) 428.0–428.9) diagnosed following hospital admission.24 The exposure of interest was serum 25(OH)D level obtained 7 to 365 days prior to the date of hospital admission and categorised as 25(OH)D <10 ng/mL; 10–19.9 ng/mL; 20–29.9 ng/mL and ≥30 ng/mL.14 We define prehospital serum 25(OH)D concentration <20 ng/mL as vitamin D inadequacy, as suggested by a recent Institute of Medicine report.25 In cases where a patient had serum 25(OH)D measured more than once in the year prior to hospitalisation, the serum 25(OH)D measured closest to the date of hospital admission was utilised.
Race was either self-determined or designated by a patient representative/healthcare proxy. Patient admission ‘type’ was defined as ‘medical’ or ‘surgical’ and incorporates the Diagnostic-Related Grouping (DRG) methodology.26 We utilised the Deyo-Charlson Index to assess the burden of chronic illness which is well studied and validated.27 Sepsis was defined by the presence of any of the following ICD-9-clinical modification (CM) codes 038.0–038.9, 020.0, 790.7, 117.9, 112.5 or 112.8128, 3 days prior to critical care initiation to 7 days after critical care initiation, a definition validated in our administrative data.7 To determine neighbourhood socioeconomic disadvantage we used geocoded residential address data29 from electronic health records; we then linked the zip+4 data to the Area Deprivation Index developed by Singh et al30 and linked to the 2000 US census by Kind et al.31
During the study period between 1998 and 2011, the chemiluminescence assay, the radioimmunoassay or liquid chromatography-mass spectroscopy (LC-MS) were employed at different times as a 25(OH)D assay method. Dates, times and type of 25(OH)D assay were recorded. The 25(OH)D assays were tested for imprecision by the clinical laboratories at the two hospitals. Imprecision testing with human serum specimens showed within-run coefficients of variation (CVs) of ≤4.5% for the chemiluminescence assay, ≤10.8% for the radioimmunoassay, and ≤8.6% for LC-MS.6
The primary end point was acute respiratory failure defined by Cooke et al24 and identified by the presence of ICD-9, codes for respiratory failure or pulmonary oedema (518.4, 518.5, 518.81 and 518.82) and mechanical ventilation (96.7×), excluding congestive heart failure (428.0–428.9) following hospital admission.24 Inclusion of mechanical ventilation codes and the exclusion of heart failure codes increases the specificity of the ICD-9 code combination for ARDS.32 In subanalyses, the acute respiratory failure end point was validated against the Berlin Definition of ARDS.33 The secondary end point was 90-day all-cause mortality obtained from the Social Security Administration Death Master File. One hundred per cent of the cohort had at least 90-day follow-up. The censoring date was 5 January 2012.
Power calculations and statistical analysis
In the cohort under study, the incidence of acute respiratory failure was 18%. By assuming acute respiratory failure incidence in patients with prehospital serum 25(OH)D >30 ng/mL to be 18% and an OR of 1.5 for incident acute respiratory failure in patients with prehospital serum 25(OH)D ≤30 ng/mL, we needed 359 patients with 25(OH)D >30 ng/mL and 359 patients with 25(OH)D ≤30 ng/mL to achieve 80% power and 5% significance.
Unadjusted associations between vitamin D groups and outcomes were estimated by bivariable logistic regression analysis. Adjusted ORs were estimated by multivariable logistic regression models with inclusion of covariate terms chosen based on the biological plausibility of possible confounding of the vitamin D—acute respiratory failure association. For the primary model, specification of each continuous covariate was adjudicated by the empiric association with the primary outcome using Akaike's Information Criterion; the overall model fit was assessed using the Hosmer Lemeshow test. Models for secondary analyses (90-day mortality) were specified identically to the primary model in order to achieve greatest analogy. Sensitivity analysis were performed for patients with 25(OH)D measured at various time points prior to hospital admission. Locally weighted scatter plot smoothing (LOWESS) was used to graphically represent the relationship between prehospital 25(OH)D concentration and rate of acute respiratory failure. A multivariable Cox’s proportional hazards model was used to illustrate the survival among patients with acute respiratory failure as vitamin D intake increases. All p values presented are two-tailed; values below 0.05 were considered to be significant. All analyses are performed using STATA 12.0MP (College Station, Texas, USA).
To validate the accuracy of ICD-9-CM and current procedural terminology (CPT)-defined acute respiratory failure assignment in our study, 206 of the 1985 cohort patients were chosen at random. Subject charts were retrospectively reviewed by two clinician investigators blinded to the subject 25(OH)D level and ICD-9-CM/CPT acute respiratory failure code assignment to determine if patients had clinical criteria for ARDS in the first 14 days of an ICU admission according to the Berlin definition: (1) acute respiratory failure not fully explained by cardiac failure or fluid overload, per the intensivist of record; (2) bilateral opacities consistent with pulmonary oedema on the chest radiograph or the CT scan; and (3) onset within 1 week after a known clinical insult or new or worsening respiratory symptoms.33 The validation criteria were met if the criteria for mild, moderate or severe ARDS, as defined by The Berlin Definition of ARDS, were achieved in the first 14 days of an ICU admission: mild if PaO2/FiO2=201–300 mm Hg, moderate if PaO2/FiO2=101–200 mm Hg and severe if PaO2/FiO2 ≤100 mm Hg with PEEP level ≥5 cm H2O was present in all ARDS cases. Diagnosis of ARDS was established by consensus of the two clinician investigators and resolved by a third in case of discrepancies.
Results
Table 1 shows demographic characteristics of the parent ICU cohort and the study cohort. Differences between the parent and study cohorts included gender, patient type and comorbidity. In the study cohort (N=1985), most patients were women, white and had medical-related DRG with a mean age of 63 years. The mean (SD) 25(OH)D in the study cohort was 29.0 (15.5) ng/mL which did not statistically differ by season of 25(OH)D draw (p=0.12, χ2). The majority of 25(OH)D measurements in the study cohort occurred within 6 months of hospital admission (20% within 1 month, 48% within 3 months and 72% within 6 months). Most study cohort patients had 25(OH)D levels ≥20 ng/mL (8% with 25(OH)D <10 ng/mL; 24% with 10–19.9 ng/mL; 24% with 20–29.9 ng/mL and 44% with ≥30 ng/mL). The 90-day mortality of the study cohort was 15.7%.
Characteristics of total ICU cohort (N=77 927) and study cohort (N=1985)
In the study cohort, the most common Major Diagnostic Codes in the cohort by system were circulatory 31.7%, digestive 14%, musculoskeletal 8.9%, kidney 8.4%, endocrine 7.7%, respiratory 4.8%, injuries 4.4% and infectious 4.2%. The mean (SD) time from hospital admission to acute respiratory failure diagnosis was 1.46 (4.43) days and the mean (SD) time from hospital admission to ICU admission was 1.90 (6.19) days. Validation of ICD-9-CM/CPT-defined acute respiratory failure assignment, as per the Berlin Definition of ARDS, showed that ICD-9-CM/CPT-defined acute respiratory failure assignment had a sensitivity of 59.4% (95% CI 46.4 to 71.5%), a specificity of 95.1% (95% CI 90.1 to 97.9%), a positive predictive value of 84.4% (95% CI 69.9 to 93.0) and a negative predictive value of 83.9% (95% CI 77.0 to 89.0).
Patient characteristics of the study cohort were stratified according to preadmission vitamin D groups (table 2). Factors that significantly differed between stratified groups included age, gender, calcium and haematocrit. Factors that did not significantly differ between stratified groups included Deyo-Charlson index, patient type (surgical vs medical) and season of 25(OH)D measurement. Table 3 indicates that age, gender and 25(OH)D are significant predictors of acute respiratory failure in our model.
Patient characteristics by prehospital vitamin D status
Multivariable-adjusted associations between covariates and acute respiratory failure
Primary outcome
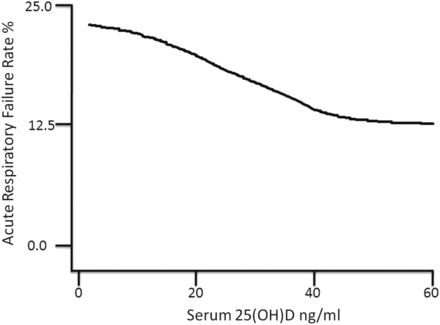
Preadmission 25(OH)D was strongly associated with acute respiratory failure during hospitalisation (table 4). The odds of acute respiratory failure was 2.1-fold, 1.7-fold and 1.5-fold higher in patients with 25(OH)D values in the <10 ng/mL, 10–19.9 ng/mL, 20–29.9 ng/mL groups, respectively, compared to those with 25(OH)D ≥30 ng/mL. 25(OH)D in the cohort remains a significant predictor of odds of acute respiratory failure following adjustment for age, race, sex, Deyo-Charlson Index and patient type (medical vs surgical). The adjusted odds of acute respiratory failure was 1.8-fold, 1.6-fold and 1.4-fold higher in patients with 25(OH)D values in the <10 ng/mL, 10–19.9 ng/mL, 20–29.9 ng/mL groups, respectively, compared to those with 25(OH)D ≥30 ng/mL (table 4). LOWESS plot (figure 1) showed a near inverse linear association between vitamin D status and an acute respiratory failure rate for 25(OH)D concentrations of approximately 40 ng/mL; beyond 40 ng/mL, the association appears flat.
Unadjusted and adjusted associations between prehospital 25(OH)D level and acute respiratory failure
Vitamin D status versus acute respiratory failure Rate. 25(OH)D=25-hydroxyvitamin D. Locally weighted scatter plot smoothing (LOWESS) utilised to represent the near inverse linear association between prehospital 25(OH)D concentration and acute respiratory failure rate. With bandwidth parameter=0.99, 1919 cohort patients were utilised to construct the curve.
Individually running the adjusted model with and without terms for assay, year, season or sepsis did not materially affect the acute respiratory failure estimates reported above (see online supplementary table S1); thus, the vitamin D-acute respiratory failure relationship was not materially confounded by these covariates. Further, utilising 25(OH)D as a continuous variable, we find that for every increase in preadmission 25(OH)D of 5 ng/mL, the odds of acute respiratory failure during hospitalisation decreases by 8% (OR 0.92, 95% CI 0.88 to 0.96; p<0.001) when adjusted for age, gender, race, type and Deyo-Charlson index.
We also performed a sensitivity analysis of the timing of prehospital 25(OH)D draw date. Sensitivity analysis of the effects of excluding patients with preadmission 25(OH)D levels obtained ≥30, ≥60, ≥90 or ≥180 days prior to admission did not materially change the association between vitamin D and ICD-9-CM defined acute respiratory failure (see online supplementary table S2). A modest attenuation of the effect size was observed as the time difference increased, but the vitamin D acute respiratory failure associations retained statistical significance.
In cohort patients with acute respiratory failure (n=381), vitamin D status was associated with risk of mortality. Though limited by sample size, patients with prehospital 25(OH)D values of <10 ng/mL, 10–19.9 ng/mL and 20–29.9 ng/mL had odds of 90-day mortality of 2.02 (95% CI 0.96 to 4.27; p=0.064), 2.57 (95% CI 1.49 to 4.45; p<0.001) and 1.57 (95% CI 0.89 to 2.80; p=0.12), respectively, compared to those with 25(OH)D ≥30 ng/mL following adjustments for age, race, sex, Deyo-Charlson Index and patient type (medical vs surgical; table 5). Cox proportional-hazards regression analysis demonstrated that in patients with acute respiratory failure, the risk of death declines with each 5 ng/mL increase in prehospital 25(OH)D adjusted for age, race, sex, Deyo-Charlson Index and patient type (medical vs surgical; HR 0.92, 95% CI 0.87 to 0.97; p=0.004).
Unadjusted and adjusted associations between prehospital 25(OH)D level and 90-day mortality in patients with acute respiratory failure
Effect modification
Analyses based on fully-adjusted models were performed to evaluate the 25(OH)D-acute respiratory failure association, and p for interaction was determined to explore for any evidence of effect modification. We individually tested for effect modification by hospital, time between 25(OH)D draw and hospital admission and season of 25(OH)D draw by adding an interaction term to the multivariate models. Effect modification analysis showed that the association between 25(OH)D and acute respiratory failure was not modified by the hospital that provided care (p interaction=0.19), or the time between 25(OH)D draw and hospital admission (p interaction=0.30). When we investigated effect modification by season of 25(OH)D draw, we identified a nearly statistically significant interaction term for 25(OH)D and acute respiratory failure (p interaction=0.07). When the cohort is analysed by restricting this to the season of 25(OH)D draw, the directionality of the association between 25(OH)D and acute respiratory failure remains, but the effect size is strongest with 25(OH)D drawn in the spring season and weakest in the fall.
Discussion
The present, two-centre study aimed to determine whether suboptimal vitamin D status prior to hospital admission was associated with acute respiratory failure in the critically ill. In unadjusted and adjusted analyses, we found increased odds of acute respiratory failure in patients with preadmission 25(OH)D <20 ng/mL. In addition, our mortality analysis suggests that patients with 25(OH)D levels <20 ng/mL before hospital admission who develop acute respiratory failure have a higher risk for mortality compared to patients with prehospital 25(OH)D levels ≥30 ng/mL.
ARDS is due to diffuse alveolar damage mediated by inflammatory cytokines such as tumour-necrosis factor, interleukin (IL)-1, IL-6 and IL-8 and subsequent neutrophil recruitment and release of oxygen free radicals that damage capillary endothelium and alveolar epithelium.34 ,35 Immunomodulatory and proinflammatory associations of vitamin D deficiency are well known. Vitamin D induces expression of the gene for cathelicidin, which promotes intracellular killing of bacteria.36 In the lung, cathelicidin likely plays a role in mucosal defence as it is produced by neutrophils, macrophages and airway epithelium, and upregulated in response to infection and inflammation.37 These biological observations indicate the potential importance of vitamin D status to innate immunity and ARDS.
Prior studies have reported robust associations of low vitamin D and sepsis, bloodstream infections and mortality in critically ill adults.13 The VITdAL-ICU trial secondary outcome data shows high-dose vitamin D supplementation reduces mortality in patients with severe vitamin D deficiency (25(OH)D≤12 ng/mL), but was not designed to study respiratory outcomes.11 In the current study, the reason for an increase in mortality in acute respiratory failure patients with vitamin D inadequacy is likely to be multifactorial. Other issues that may be important for critical illness outcomes include vitamin D related effects on vascular endothelial growth factor, endothelin and the renin-angiotensin-aldosterone system.38–40 Further, comorbidities including incident hypertension, glucose intolerance, the metabolic syndrome, obesity and cardiovascular disease are all associated with low 25(OH)D and higher mortality.41–45
The present study is not without potential limitations. Observational studies may be limited by bias, confounding, and/or reverse causation.46 Importantly, causality cannot be determined in our study. Our utilisation of ICD-9 and CPT coding likely reflects the measured incidence of acute respiratory failure in the cohort rather than the actual incidence. Ascertainment bias may exist in our study as the patient cohort under study had vitamin D status measurements for reasons that may be absent in other critically ill patients. Despite adjustment for multiple potential confounders, residual confounding may contribute to the observed differences in outcomes and vitamin D status could simply be a marker of baseline healthy behaviours. Specifically, we are unable to adjust for immobilisation, excessive alcohol intake, smoking status, genetic factors, hypertension, low-density lipoprotein-cholesterol, education level and low milk consumption all of which can alter 25(OH)D.47 ,48 The percentage of cohort patients who are female is 55% while the percentage of African-Americans is only 6%, which may limit generalisability. The 25(OH)D-mortality association appears to be preserved when 25(OH)D is obtained within 30 days of admission. Despite this observation, vitamin D levels at the time of hospitalisation may be different from the levels when prehospital values were drawn. We also do not have any information as to why 25(OH)D concentrations were obtained in the cohort. Finally, our observations may represent a healthy user effect rather than causality.49
The present study has several strengths. To the best of our knowledge, our study is the first large sample study to evaluate an association between prehospital vitamin D and incident acute respiratory failure. Crucially, we have sufficient statistical power to detect a clinically relevant difference in acute respiratory failure between the vitamin D groups. The exclusion of ICD-9 codes related to heart failure and requirement of mechanical ventilation ICD-9 codes for our exposure increases the specificity of the codes for ARDS.24 The Deyo-Charlson Index allowed us to account for chronic medical comorbidities. Finally, by measuring vitamin D status at least 7 days prior to hospitalisation, we attempted to uncouple the influence of acute illness and inflammation on 25(OH)D levels.
Conclusion
In summary, prehospital vitamin D inadequacy (25(OH)D <20 ng/mL) is associated with incident acute respiratory failure during critical illness and death in patients with acute respiratory failure. Despite our observations, supplementation of vitamin D in critically ill patients cannot be advocated for prevention or treatment of acute respiratory failure as our study does not establish causation. The data presented in combination with the hypothesis generating VITdAL-ICU trial data11 does provide an impetus to perform randomised controlled trials to determine whether vitamin D supplementation therapy might have benefit in decreasing the incidence of or improving outcomes of ARDS in critically ill patients with low 25(OH)D.
References
Footnotes
Acknowledgements This manuscript is dedicated to the memory of our dear friend and colleague Nathan Edward Hellman, MD, PhD. The authors thank Shawn Murphy and Henry Chueh and the Partners HealthCare Research Patient Data Registry group for facilitating use of their database.
Contributors DRT and KBC designed research. DRT, TM and KBC conducted research. KBC, KMM and FKG provided essential materials. KBC performed statistical analysis. DRT, TM, AAL, KA, SAQ, KAL-S, KMM, SWP, FKG, CAC, Jr, EG and KBC wrote the paper. KBC had primary responsibility for final content. All authors read and approved the final manuscript.
Competing interests None declared.
Ethics approval Partners HealthCare Human Research Committee (Institutional Review Board).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.