Article Text
Abstract
The growing utilisation of indwelling pleural catheters (IPCs) has put forward a new era in the management of recurrent symptomatic pleural effusions. IPC use is safe compared to talc pleurodesis, though complications can occur. Pleural infection affects <5% of patients, and is usually responsive to antibiotic treatment without requiring catheter removal or surgery. Pleural loculations develop over time, limiting drainage in 10% of patients, which can be improved with intrapleural fibrinolytic therapy. Catheter tract metastasis can occur with most tumours but is more common in mesothelioma. The metastases usually respond to analgaesics and/or external radiotherapy. Long-term intermittent drainage of exudative effusions or chylothorax can potentially lead to loss of nutrients, though no data exist on any clinical impact. Fibrin clots within the catheter lumen can result in blockage. Chest pain following IPC insertion is often mild, and adjustments in analgaesics and drainage practice are usually all that are required. As clinical experience with the use of IPC accumulates, the profile and natural course of complications are increasingly described. We aim to summarise the available literature on IPC-related complications and the evidence to support specific strategies.
- Pleural Disease
- Empyema
- Mesothelioma
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Pleural effusions are common worldwide, with an estimated 1.5 million new cases in the USA and 250 000 in the UK a year.1 Malignant pleural effusion (MPE) is the commonest cause of recurrent exudative effusions and many patients suffer from recurrent symptomatic fluid accumulation that requires repeated drainages. Consequently, MPE presents a major healthcare burden. In Western Australia, in-patient care of MPE costs approximately US$6 million per million population annually.2
Talc pleurodesis has been, for many decades, the preferred treatment for MPEs. Data from recent years, however, have raised doubts on its efficacy and safety.3 The indwelling pleural catheter (IPC) provides a revolutionary alternative for achieving long-term control of recurrent effusions, especially MPEs. Increasing centres worldwide now employ IPCs instead of talc pleurodesis as the default management for MPEs. An estimated 40 000 patients are treated with IPCs each year in the USA alone.
IPCs are silicon tubes, tunnelled and secured subcutaneously (with a pro-fibrotic cuff), that end with a one-way valve. This catheter allows ambulatory effusion drainage by the patients or their carers after minimal training. Its principle aim is to provide symptom relief instead of creating pleural symphysis. Cumulative evidence proposes potential advantages of IPCs over talc pleurodesis.2 ,4
First, pleurodesis is only useful in patients with fully expanded lungs after fluid evacuation. It is increasingly realised that non-expandable (or ‘trapped’) lungs are common in MPEs.5 A recent study using pleural ultrasound as a screening tool found 50–60% of MPE patients had non-expandable lungs and poor performance not suitable for talc pleurodesis.6 IPC, however, can be used for all MPE patients whether the lung is expandable or trapped. The improvement in dyspnoea and quality of life in patients treated with IPC is comparable to talc pleurodesis up to 6 months, after which IPC may be superior in relieving dyspnoea.7
Second, even in those suitable for pleurodesis, Dresler et al8 (n=482) showed that successful fluid control was only achieved in approximately 70% of patients by 1 month irrespective of whether the talc was delivered by thoracoscopic insufflation (poudrage) or as a slurry via chest tubes. Pleurodesis failure progressively increased with prolonged survival. By 6 months, talc pleurodesis had failed in approximately 50% of patients.8 Fysh et al3 showed, in 165 patients with mesothelioma, that talc pleurodesis was successful in life-time control of MPEs in about one-third of patients; 32% of all patients required further pleural intervention. On the contrary, over 90% of patients treated with IPCs did not need further drainages.9 The additional emotional stress from recurrent symptoms and invasive pleural drainages significantly jeopardises patients’ quality of life (QoL).
Third, talc pleurodesis requires hospitalisation, often for 4–5 days, whereas IPC is often inserted as a day-case procedure.3 ,10 Two randomised trials have shown significantly shorter initial admission time for IPC than pleurodesis.7 ,11 A pilot non-randomised study suggested that patients treated with IPC spent significantly fewer days in hospital (for any causes) from procedure to death than those pleurodesed.12 This is being verified in a randomised trial.13 If confirmed, this benefit will influence treatment choice in this patient population, whose median survival rate is only 4–6 months.
Fourth, pleurodesis is known to provoke intense pleural and systemic inflammation, with a median rise in blood C reactive protein of 360% from baseline.14 The resultant pain and fever can be severe. Talc pleurodesis can cause hypoxaemia and, in severe cases, acute respiratory failure.15 Management of IPC avoids these disadvantages.
An increasing number of MPE patients are treated with IPC either as a first-line option instead of pleurodesis, or as rescue therapy when pleurodesis fails. The indications of IPC have also been extended to benign recurrent effusions such as hepatic hydrothorax, cardiac failure and chylothorax.16–20 Clinicians must be adequately equipped to handle common complications associated with IPC use, which is reported to occur in 10–20% of patients.9 ,21 ,22 There are complications, not specific to IPCs, associated with small bore catheter insertion using the Seldinger technique, for example, wound infection or cellulitis, bleeding, organ injury, pneumothorax, dislodgement, etc, which have been reviewed elsewhere.23
There are, however, several important, albeit uncommon, complications that are peculiar to IPC use. We review the current evidence on the occurrence, risk factors and management of these IPC-related complications.
IPC-related pleural infection
The standard diagnostic criteria for pleural infection include the relevant clinical signs and symptoms of infection, presence of low pleural fluid pH (and/or glucose), or presence of pus or bacteria in the pleural fluid.24 In patients fitted with an IPC, the diagnosis of pleural infection can be challenging. Malignant effusions often have low pleural fluid pH and high lactate dehydrogenase, and fever related to underlying tumour is not uncommon. Akin to long-term urinary catheter use, bacterial ‘colonisation’ can occur in IPC-treated patients whose pleural fluid yields positive microbiology but without clinical manifestation of empyema or the typical biochemical profile of infected pleural fluid. There are no published studies investigating the incidence, bacteriology and significance of bacterial colonisation in IPC-treated patients.
Pleural infection associated with long-term IPC use is of concern to clinicians, especially oncologists administering chemotherapy. The incidences of IPC-related pleural infection in reported (usually small) series range from 0% to 12% (table 1). A large multicentre review characterised 1021 patients with IPC from 11 centres in Europe, North America and Australasia, and found an infection rate of only 4.8%.25
A summary of studies reporting the rate of IPC-related pleural infection (in chronological order)
Cutaneous flora, including Staphylococcus spp (especially S. aureus), accounts for most of the reported cases, followed by Pseudomonas aeruginosa and Enterobacteriaceae.25 IPC-related pleural infection typically occurs at least 6–8 weeks after insertion. Such lag time in the occurrence of IPC-related pleural infection speaks against any direct relation to the insertion procedure. Studies investigating the mechanisms leading to pleural infection in the setting of IPC are lacking. Bacteria colonising the skin can potentially migrate to the pleura along the IPC or its tract over time, or else, lung parenchymal infection may allow entry of bacteria into the pleural cavity. These data therefore do not support a role of routine prophylactic antibiotics at insertion. Instead, they highlight the importance of catheter aftercare, including education of patients and their carers as well as community support.
IPC-related pleural infections have generally been mild. In one study, the overall mortality from pleural infection was only 0.29% in all IPC-treated patients.25 This is exceptionally low compared with reported mortality in large series of community-acquired pleural infection (in patients without IPC and underlying cancer).26 Most cases resolved with antibiotic treatment. None of the 50 cases in the aforementioned series required surgery.25 More than one-fourth were managed as outpatients, with oral antibiotics. Removal of the IPC is not necessary unless the infection fails to respond. The IPC provides ready access to drainage of infected material and may explain the low mortality; it also permits intrapleural tissue plasminogen activator/DNase therapy, which was used in 26% of the 50 patients in the series by Fysh et al,25 to facilitate drainage.26 Most patients with IPCs also receive regular medical assessments, which may allow early detection of infections.
Pleurodesis is common after IPC-related pleural infection and, in one study, allowed removal of the catheter in 62% of patients (80% in those with S. aureus empyema).25 This echoed the proof-of-concept data using staphylococcal enterotoxin as a pleurodesing agent.27 Pilot pooled data from the UK suggested an association with longer survival in MPE patients who had pleural infection.28 Indeed, bacterial bioproducts from S. aureus have halted mesothelioma growth in animal studies.29 This interesting concept warrants further exploration.
Reassuring data of IPC use in immunocompromised patients are mounting. A recent series of patients with underlying haematological malignancies treated with IPCs reported infection and mortality rates of 7% and 2%, respectively, despite the high background risks of ongoing chemotherapy and cytopaenia.30 A retrospective cohort study from the Mayo clinic also found no significant differences in the rate of IPC-related pleural infection comparing patients with or without ongoing chemotherapy.31 A series from Oxford, UK, included 23 IPC-treated patients who received chemotherapy (with transient neutropaenia) and similarly reported no significant increase in risks of infection.32
Data on IPC use in treating benign effusions are also promising. In a multicentre study reporting the use of IPC in 57 non-malignant effusions, suspected pleural infection occurred only in two cases (3.5%); both had negative pleural fluid culture and IPC removed subsequent to the suspected infection.16 In a Canadian series using IPCs (n=43) in patients with cardiogenic pleural effusions, no pleural infection was found.17
The low incidence (and hence small number of patients) of pleural infection has precluded multivariate analyses for risk factors. Potentially predisposing factors to IPC-related pleural infection that warrant further investigations are listed in table 2. The tunnelled tract and cuff are designed in part to reduce entry of pathogens into the pleural space, analogous to long-term catheters for dialysis or vascular access.33 In addition, the one-way valve of the IPC allows unidirectional fluid flow and theoretically minimises ascending infection into the pleural cavity. Catheters that cease draining with little residual pleural fluid should be promptly removed. A recent prospective study reported the effectiveness of a quality improvement intervention (preoperative antibiotics, full sterile draping and limiting placement to a single defined location) in reducing the rates of IPC-related infection.34
Potential risk factors for IPC-related pleural infection
Catheter tract metastasis
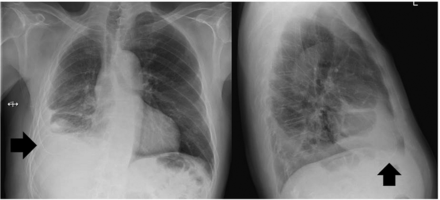
Patients with catheter tract metastasis (CTM) typically present with a new, and often painful, subcutaneous nodule/mass near the IPC insertion site or its subcutaneous tract. The lesions can often be recognised on CT imaging as soft tissue opacity at typical sites initially resembling scarring and, later, nodularity with or without peripheral invasion42 (figure 1). Histological confirmation should be pursued if there is concern over the diagnosis, as mimics of CTM do exist.43
CT images of a patient with mesothelioma who developed catheter tract metastasis around his indwelling pleural catheter (IPC), which was in place for 5 months. The pain over the tumourous growth (arrow) was partially relieved with analgaesics, and he subsequently underwent external beam irradiation to the catheter tract metastasis, with good symptomatic control. His IPC was kept for drainage of malignant effusion.
The reported incidences of CTM have varied widely with an average figure of below 5% in earlier studies.23 CTM was only reported in 1 of the 52 IPC-treated patients in a randomised study.7 On the contrary, it affected up to 10% of patients in a recently reported cohort of which 60% were patients with mesothelioma.42 The variations in incidences could be related to the difference among primary malignancies in the studies, definition of CTM and/or a difference in awareness of CTM over the years. Most CTMs developed late (median 280 days) after IPC insertion.42
The aetiology of CTM is still largely unknown. One hypothesis is that tumour cells grow along the puncture points at the parietal pleura, to adjacent subcutaneous tissue, and the presence of a long-term catheter may encourage inflammation and vascularisation along the tract, which can potentially be the nidus for tumour spread. Mesothelioma is known for its propensity to spread along pleural puncture tracts and accounts for the majority of cases in the two studies on IPC-related CTM.42 ,44 However, CTM from other cancers, such as lung, breast and ovary, has also been reported.
Patients with CTM can usually be treated effectively with simple analgaesics and external beam radiotherapy without the need to remove the IPC. The role of prophylactic radiotherapy in reducing postprocedural tract metastases in mesothelioma remains controversial with three small randomised trials showing contrasting results.45 ,46 CTM has been reported to develop despite prophylactic radiotherapy after IPC insertion.44 IPCs present an ongoing threat of tumour spread, unlike other one-off pleural procedures. It is therefore difficult to foresee a role from a single course of postinsertion radiotherapy. Two large multicentred randomised trials (PIT and SMART) in the UK are now investigating the role of prophylactic radiotherapy after pleural interventions,47 with at least one including a specific subgroup on IPCs.
Symptomatic loculations
The presence of an IPC could facilitate pleural symphysis or ‘spontaneous pleurodesis’ in approximately 40% of patients.9 ,16 It has been hypothesised that the catheter may induce fibrin deposition within the pleural cavity, and/or that regular drainage keeps the pleural space dry allowing apposition of the visceral and parietal pleura. However, fibrin deposition, whether stimulated by the IPC or underlying tumour, can also unfavourably induce septations and pleural fluid loculation, thus limiting effective IPC drainage.5
IPC-related symptomatic loculation is defined by residual pleural effusion that fails to evacuate through a patent IPC, breathlessness related to the effusion and absence of evidence of pleural infection.48 It is reported to be present in 5–14% of IPC-treated patients, and typically occurs at about 2 months after IPC insertion.7 ,9 ,12 ,17 ,48 ,49 Pleural aspiration, removal of the ineffective IPC and insertion of a second catheter targeting the residual locules can be considered. These strategies necessitate invasive procedures with inherent risks, and the feasibility depends on the locations and sizes of the residual locules.49
Intrapleural fibrinolysis provides a feasible alternative. This may help to lyse adhesions, restores drainage via IPC and thus avoids a second invasive pleural procedure (figure 2). A recent four-centre retrospective study examined 66 patients with IPC insertion for MPE, who received intrapleural fibrinolytic therapy for symptomatic loculations.48 Symptomatic response and improvement in pleural fluid drainage were achieved in the majority of the patients (83% and 93%, respectively) (figure 2), with corresponding reduction in residual pleural fluid on chest X-ray.48 Pleural bleeding occurred in two (3%) patients; both responded to supportive blood transfusion without haemodynamic consequences or need of invasive interventions. No systemic bleeding was reported. The efficacy and safety profile of intrapleural fibrinolytic therapy is encouraging, but heterogeneity in its use and the high recurrence rate of symptomatic loculation (40%)48 clearly call for further clinical trials to guide patient selection and optimise the delivery regimen of fibrinolytics.
This patient with a malignant pleural effusion was treated with an indwelling pleural catheter (IPC). After 4 months of drainage, he developed symptomatic loculations. The fluid could not be evacuated despite a patent catheter in the right pleural cavity. He was given intrapleural instillation of tissue plasminogen activator (tPA), with excellent effect. The post-treatment chest radiograph revealed an underlying trapped lung and a significant pleural rind of tumour.
Nutrition and cell loss
Pleural fluid contains essential nutrients such as proteins, fat and glucose, and white cells. Each litre of exudative fluid typically contains over 30 g of protein. There have been concerns that long-term intermittent drainage potentiates nutrient loss and immunological impairment, especially for exudative effusion or chylothorax. It is difficult to compensate for the nutritional loss with increased diet intake in malnourished patients with end-stage malignancy. Malnutrition and cachexia is increasingly recognised to affect the outcomes of patients with cancer.50 Similar concerns have been expressed in the use of IPC for benign effusions where prolonged drainage is expected.16 ,51
Currently, data reporting the effect of IPC drainage on nutrition and immunology are scant. A case series including 11 benign chylothoraces managed by IPC drainage found no observable changes in nutrition and blood cell counts, though the small sample size, missing data and absence of comparison with a control group precluded the drawing of firm conclusions.18 Another retrospective study of malignant chylothoraces found no significant changes in body weight and absolute lymphocyte counts.52 However, a decline in serum albumin level (median drop 0.2 g/dL) pre-IPC and post-IPC at median follow-up of 6 months after the procedure was noted, which was reversible with IPC removal.52 No difference in the rate of protein depletion was noted between the groups receiving IPC versus talc pleurodesis in a prospective 12-month study.12 Prospective cohort studies with longer follow-up interval are required before definite conclusions can be drawn. In the meantime, physicians should be aware of the potential effects on nutrition status, in particular for those on prolonged IPC drainage with high protein content and voluminous output.
Fracture of catheters on removal
Spontaneous pleurodesis can develop in 40–70% of patients with IPC in situ, which permits catheter removal.5 On other occasions, the IPC may be removed due to cessation of drainage or development of serious complications such as empyema or severe pain. Removal of the catheter requires freeing the cuff from the often tight fibrinous adhesions anchoring the shaft and cuff of the catheter to the surrounding tissue. Fracture of catheters during removal, though rare, can occur especially when traction force is applied. In a two-centre series on 61 IPC removals, 10% (6 cases) were complicated by fracture of the catheter or required iatrogenic severing because of difficulty in removal53 (figure 3). On subsequent follow-up, none suffered from any complications as a result of the retained IPC, including two patients who received chemotherapy.53 The study suggests that aggressive attempts at removing the retained fragments are unnecessary.
Chest radiograph of a patient with mesothelioma and indwelling pleural catheter (IPC) for palliation of right pleural effusion. The drainage output reduced with minimal residual effusion after 10 months. Attempt was made to remove the IPC en bloc, but the part distal to the cuff adhered tightly to underlying tissue after freeing the cuff. A decision was made to sever the catheter close to the pleura. The IPC fragment (arrow) was retained without any problems in subsequent follow-up.
Catheter blockage
The formation of dense fibrinous tissue around and within the IPC can occasionally lead to blockage of some lumen, but complete occlusion of all lumens by materials is uncommon, with incidence <5%23 (figure 4). Saline flush and manipulation along the catheter may dislodge occluding materials and re-establish patency. Non-draining blocked catheters with little residual pleural fluid should be removed to prevent infection.
An indwelling pleural catheter (IPC) removed from a patient with mesothelioma. On inspection after removal, the lumen of the catheter was blocked by a string of fibrin clot (arrow), which could be pulled out intact from the catheter using a pair of forceps.
Chest pain
Mild pain during or immediately after IPC insertion is common (36%), and can be relieved with the use of longer acting local anaesthetics and conscious sedation.23 During intermittent drainage under suction, negative pressure may develop inside the pleural cavity whether or not the underlying lung is trapped, resulting in pain. This can be alleviated by slowing or stopping the drainage. Severe pain requiring catheter removal is rare (0.6%) in the published literature.23
Cost of IPC management
Costs related to IPC management can be (1) direct, for example, that related to initial IPC insertion and subsequent drainage (kits, personnel support for drainage assistance) and (2) indirect, for example, that related to management of IPC complications. Several factors can significantly affect the overall cost of IPC management, including (1) healthcare and insurance systems, (2) setting of IPC insertion and removal, for example, inpatient versus outpatient, (3) duration of IPC in situ, which in turn depends on survival and rates of spontaneous pleurodesis, (4) complication rates, for example, need for antibiotic therapy for infections, radiotherapy for catheter tract metastases, (5) drainage regimes and frequency, for example, daily versus symptom-guided and (6) need for additional community support, for example, family member/carer versus nurse-assisted drainage, frequency of medical review, etc. These factors vary widely in the individual patient and make a thorough cost-analysis of IPC treatment or direct comparison with pleurodesis extremely difficult. In general, costs of IPC treatment will increase as the IPC is in situ for longer, with associated greater requirements for drainage consumables and nursing resources, and higher risk of complications.54 These factors mean that there will be no simple answer to the cost-effectiveness of IPC against pleurodesis.
Most studies to date on IPC cost-analysis are retrospective and used indirect comparisons with conventional treatments; and findings from one healthcare setting often cannot be extrapolated to others. A retrospective Dutch study found that the direct costs of IPC and drainage consumables were comparable to the estimated hospitalisation costs for pleurodesis, but did not include indirect costs.55 Early hospital charges were lowest when the IPC was inserted on an outpatient basis, compared with inpatient IPC insertion or doxycycline pleurodesis, however, long-term hospital charges did not differ significantly.35 IPC was more cost-effective than pleurodesis in patients with limited (<6–12 weeks) survival compared to those who survived >12 months.56 ,57 Hence, IPC treatment for mesothelioma was four times more expensive than that for lung cancer effusions. However, these projected figures are debateable, and may possibly not be extrapolated to other healthcare settings.
Data collected from the TIME-2 randomised controlled study found no significant difference in the overall cost of IPC versus talc pleurodesis.58 IPC costs were significantly lower in patients with limited survival (<14 weeks). Study limitations included potential selection bias, as only patients with an expected survival of >3 months were included in the original study. Also, cost saving was lost when >2 h/week of community nursing support was required. Again, the costs of individual items will vary among countries and extrapolation to other systems may not be appropriate.
Future directions
The growing recognition of IPC as effective palliative therapy for pleural effusions worldwide means that more catheters will be inserted and thus clinicians will be managing more IPC-related complications in coming years. However, many knowledge gaps and heterogeneity in management exist among different centres, which highlight the lack of robust evidence in guiding a more united approach to the prevention and management of IPC-related complications. Future studies should focus on identifying risk factors of common complications and understanding their pathobiology in order to optimise patient outcomes. Studies reporting the longer term outcomes of patients implanted with IPC for benign pleural effusion are also needed.
References
Footnotes
Funding YCGL is a National Health & Medical Research Council (NHMRC) Career Development Fellow and has received research project grant funding from the NHMRC, New South Wales Dust Disease Board, Sir Charles Gairdner Research Advisory Committee, Westcare and the Cancer Council of Western Australia. Dr Thomas has received research scholarship support from NH&MRC, WA Cancer and Palliative Care Network, and Institute of Respiratory Health. MMSL has received support from Ho Hung Chiu Medical Education Foundation Fellowship for attachment at Sir Charles Gairdner Hospital, WA.
Competing interests YCGL and RT are investigators of AMPLE-2, a multicentre randomised trial for which Rocket Medical provides free drainage equipment for participants. YCGL has served on the advisory board of CareFusion and Sequana Med Ltd and has received an unrestricted educational grant from Rocket Medical UK for clinical research.
Provenance and peer review Commissioned; externally peer reviewed.
Data sharing statement No additional data are available.