Abstract
Background and Objectives
Aclidinium bromide is a long-acting muscarinic antagonist approved for the long-term maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD). This 12-week phase III study evaluated efficacy and tolerability of aclidinium 200 or 400 μg in patients with moderate-to-severe COPD.
Methods
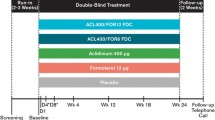
In this double-blind study, 544 patients with COPD were randomized to placebo or twice-daily aclidinium 200 or 400 μg administered by Genuair®/Pressair®. Lung function, health status [measured by the St. George’s Respiratory Questionnaire (SGRQ)], dyspnea [measured using the Transition Dyspnea Index (TDI)], and safety were assessed throughout the study.
Results
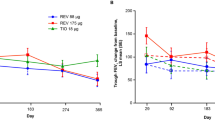
Mean changes from baseline in morning trough forced expiratory volume in 1 s (FEV1) at week 12 (primary endpoint) were significantly higher for aclidinium than for placebo (200 μg, 51 mL; 400 μg, 72 mL; both p < 0.05). Aclidinium also significantly improved other lung function outcomes. At week 12, improvements from baseline were observed with aclidinium in SGRQ total score (200 μg, −6.0; 400 μg, −5.4) and TDI focal score (200 μg, 1.0; 400 μg, 1.3). Furthermore, clinically important improvements in SGRQ total and TDI focal scores were achieved by 45 and 51 % of patients, respectively, who received aclidinium 400 μg, with a significant difference versus placebo for TDI (p < 0.05). Anticholinergic-related adverse events (e.g., dry mouth) were infrequent, occurring <2 % for any event in any treatment group. Both aclidinium doses were well tolerated.
Conclusion
This study demonstrates efficacy and safety of aclidinium in COPD patients. Unexpected baseline imbalances between treatment groups may have impacted the aclidinium treatment benefit in this study.




Similar content being viewed by others
Notes
Registered trademarks of Almirall, SA, Barcelona, Spain for use within the European Union, Iceland, Norway, and Switzerland as Genuair® and within the USA as Pressair® .
References
World Health Organization (WHO). The global burden of disease: 2004 update. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf. Accessed 21 Dec 2011.
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
DiBonaventura M, Paulose-Ram R, Su J, et al. The impact of COPD on quality of life, productivity loss, and resource use among the elderly United States workforce. COPD. 2012;9(1):46–57.
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65.
Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311(7):421–5.
Sentellas S, Ramos I, Alberti J, et al. Aclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: in vitro plasma inactivation and pharmacological activity of its main metabolites. Eur J Pharm Sci. 2010;39(5):283–90.
Kerwin EM, D’Urzo AD, Gelb AF, et al. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD. 2012;9(2):90–101.
Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40(4):830–6.
Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–9.
Mahler DA, Witek TJ Jr. The MCID of the Transition Dyspnea Index is a total score of one unit. COPD. 2005;2(1):99–103.
Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2(1):111–24.
Koch GG, Amara IA, Davis GW, et al. A review of some statistical methods for covariance analysis of categorical data. Biometrics. 1982;38(3):563–95.
Koch GG, Gillings DB, Stokes ME. Biostatistical implications of design, sampling, and measurement to health science data analysis. Annu Rev Public Health. 1980;1:163–225.
Koch GG, Tangen CM, Jung JW, et al. Issues for covariance analysis of dichotomous and ordered categorical data from randomized clinical trials and non-parametric strategies for addressing them. Stat Med. 1998;17(15–16):1863–92.
Miratrix LW, Sekhon JS, Yu B. Adjusting treatment effect estimates by post-stratification in randomized experiments. 2012. http://sekhon.berkeley.edu/papers/postadjustment.pdf. Accessed 3 Apr 2012.
Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–69.
Mahler DA, Tomlinson D, Olmstead EM, et al. Changes in dyspnea, health status, and lung function in chronic airway disease. Am J Respir Crit Care Med. 1995;151(1):61–5.
Hajiro T, Nishimura K, Tsukino M, et al. A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPD. Chest. 1999;116(6):1632–7.
Witek TJ Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J. 2003;21(2):267–72.
Calverley PM, Rennard SI. What have we learned from large drug treatment trials in COPD? Lancet. 2007;370(9589):774–85.
Westwood M, Bourbeau J, Jones PW, et al. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res. 2011;12:40.
O’Donnell DE, Guenette JA, Maltais F, et al. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012;141(3):753–62.
Guenette JA, Webb KA, O’Donnell DE. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J. 2012;40(2):322–9.
Hannink JD, van Helvoort HA, Dekhuijzen PN, et al. Dynamic hyperinflation during daily activities: does COPD Global Initiative for Chronic Obstructive Lung Disease stage matter? Chest. 2010;137(5):1116–21.
Fuhr R, Magnussen H, Sarem K, et al. Efficacy of aclidinium bromide 400 microgram twice daily compared with placebo and tiotropium in patients with moderate-to-severe COPD. Chest. 2012;141(3):745–52.
Singh D, Magnussen H, Kirsten A, et al. A randomised placebo- and active-controlled dose-finding study of aclidinium bromide administered twice a day in COPD patients. Pulm Pharmacol Ther. 2012;25(3):248–53.
Acknowledgments
The authors would like to thank the ACCORD II study investigators. The authors would also like to thank Gary Koch, PhD and Paul Jones, PhD, FRCP for helpful discussions. Editorial assistance by Joy Ramos, PhD of Prescott Medical Communications Group (Chicago, IL, USA) was funded by Forest Research Institute (FRI).
Funding
This study was funded by FRI, a wholly owned subsidiary of Forest Laboratories, Inc., and Almirall, S.A.
Author Contributions
Stephen I. Rennard, Paul D. Scanlon, Gary T. Ferguson, and Ludmyla Rekeda contributed to the analysis and interpretation of data, critically revised the manuscript for important intellectual content, and provided final approval of the manuscript. Brian T. Maurer, Esther Garcia Gil, and Cynthia F. Caracta contributed to the design of the study and analysis and interpretation of data, critically revised the manuscript for important intellectual content, and provided final approval of the manuscript.
Disclosures
Stephen I. Rennard has received honoraria for lectures from AARC, Almirall, Am Col Osteopathic Physicians, Asan Medical Center, American Thoracic Society, California Society of Allergy, CME Incite, COPD Foundation, Creative Educational Concepts, Dey, Duke University, Forest, France Foundation, HSC Medical Education, Information TV, Lung Association, Novartis, Horsham, Nycomed, Otsuka, PeerVoice, Pfizer, Shaw Science, University of Washington, University of Alabama Birmingham, VA Sioux Falls. Stephen I. Rennard has also received honoraria for consulting with the following: ABIM, Able Associates, Adelphi Research, Align2Action, Almirall, APT Pharma/Britnall, Astra-Zeneca, American Thoracic Society, Beilenson, Boehringer Ingelheim, Boehringer Ingelheim (ACCP), BoomCom, Britnall and Nicolini, Capital Research, Chiesi, Clarus Acuity, CommonHealth, Complete Medical Group, Consult Complete, COPDForum, DataMonitor, Decision Resources, Dunn Group, Easton Associates, Equinox, Forest, Frankel Group, Fulcrum, Gerson Lehman, Globe Life Sciences, Guidepoint, Health Advanced, Hoffman LaRoche, Informed, Insyght, KOL Connection, Leerink Swan, M. Pankove, McKinsey, MDRxFinancial, Medimmune, Merck, Novartis, Nycomed, Oriel, Osterman, Peal, Penn Technology, Pennside, Pfizer, PharmaVentures, Pharmaxis, Prescott, Price Waterhouse, Propagate, Pulmonary Reviews, Pulmatrix, Reckner Associates, Recruiting Resource, Roche, Sankyo, Schering, Schlesinger Medical, Scimed, Smith Research, Sudler and Hennessey, Summer Street Research, Talecris, Think Equity, UBC, Uptake Medical, Vantage Point Management. Paul D. Scanlon has conducted research for Boehringer Ingelheim, Forest Laboratories, Inc., GlaxoSmithKline, Pearl Therapeutics, Pfizer Inc., and Novartis and has served on scientific advisory committees for GlaxoSmithKline and Merck. Gary T. Ferguson has provided consulting services, participated in advisory boards, served as speaker, and conducted research for various pharmaceutical companies including Forest Laboratories, Inc., GlaxoSmith Kline, Boehringer Ingelheim, AstraZeneca, Pearl Therapeutics, Sunovion, and Novartis. Ludmyla Rekeda, Brian T. Maurer, and Cynthia F. Caracta are employees of FRI. Esther Garcia Gil is an employee of Almirall, SA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov identifier: NCT01045161.
On behalf of the ACCORD COPD II Study Investigators.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rennard, S.I., Scanlon, P.D., Ferguson, G.T. et al. ACCORD COPD II: A Randomized Clinical Trial to Evaluate the 12-Week Efficacy and Safety of Twice-Daily Aclidinium Bromide in Chronic Obstructive Pulmonary Disease Patients. Clin Drug Investig 33, 893–904 (2013). https://doi.org/10.1007/s40261-013-0138-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0138-1