Abstract
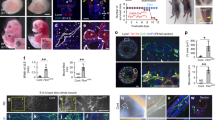
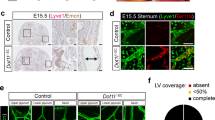
Lymphatic vessels are essential for immune surveillance, tissue fluid homeostasis and fat absorption. Defects in lymphatic vessel formation or function cause lymphedema. Here we show that the vascular endothelial growth factor C (VEGF-C) is required for the initial steps in lymphatic development. In Vegfc−/− mice, endothelial cells commit to the lymphatic lineage but do not sprout to form lymph vessels. Sprouting was rescued by VEGF-C and VEGF-D but not by VEGF, indicating VEGF receptor 3 specificity. The lack of lymphatic vessels resulted in prenatal death due to fluid accumulation in tissues, and Vegfc+/− mice developed cutaneous lymphatic hypoplasia and lymphedema. Our results indicate that VEGF-C is the paracrine factor essential for lymphangiogenesis, and show that both Vegfc alleles are required for normal lymphatic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rossant, J. & Howard, L. Signaling pathways in vascular development. Annu. Rev. Cell Dev. Biol. 18, 541–573 (2002).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Rennert, P.D., Browning, J.L., Mebius, R., Mackay, F. & Hochman, P.S. Surface lymphotoxin a/b complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 184, 1999–2006 (1996).
Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
Futterer, A., Mink, K., Luz, A., Kosco-Vilbois, M.H. & Pfeffer, K. The lymphotoxin b receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9, 59–70 (1998).
Sabin, F.R. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1, 367–391 (1902).
van der Putte, S.C.J. The early development of the lymphatic system in mouse embryos. Acta Morphol. Neerl.-Scand. 13, 245–286 (1975).
Oliver, G. & Detmar, M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 16, 773–783 (2002).
Wigle, J.T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 (1999).
Wigle, J.T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505–1513 (2002).
Petrova, T.V. et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21, 4593–4599 (2002).
Hong, Y.K. et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 225, 351–357 (2002).
Dumont, D.J. et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 282, 946–949 (1998).
Kaipainen, A. et al. Expression of the fms-like tyrosine kinase FLT4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 92, 3566–3570 (1995).
Makinen, T. et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 20, 4762–4773 (2001).
Jeltsch, M. et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276, 1423–1425 (1997).
Veikkola, T. et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 6, 1223–1231 (2001).
Karkkainen, M.J. et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 25, 153–159 (2000).
Joukov, V. et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 16, 3898–3911 (1997).
Achen, M.G. et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 95, 548–553 (1998).
Saaristo, A. et al. Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. FASEB J. 16, 1041–1049 (2002).
Stacker, S.A. et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J. Biol. Chem. 274, 32127–32136 (1999).
Kukk, E. et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 122, 3829–3837 (1996).
Prevo, R., Banerji, S., Ferguson, D.J.P., Clasper, S. & Jackson, D.G. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem. 276, 19420–19430 (2001).
Breiteneder-Geleff, S. et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 154, 385–394 (1999).
Schacht, V. et al. T1a/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 22, 3546–3556 (2003).
Baldwin, M.E. et al. The specificity of receptor binding by vascular endothelial growth factor-D is different in mouse and man. J. Biol. Chem. 276, 19166–19171 (2001).
Shalaby, F. et al. Failure of blood island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62–66 (1995).
Avantaggiato, V., Orlandini, M., Acampora, D., Oliviero, S. & Simeone, A. Embryonic expression pattern of the murine figf gene, a growth factor belonging to platelet-derived growth factor/vascular endothelial growth factor family. Mech. Dev. 73, 221–224 (1998).
Karkkainen, M.J. et al. A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. USA 98, 12677–12682 (2001).
Yuan, L. et al. Abnormal lymphatic vessel development in neuropilin-2 mutant mice. Development 129, 4797–4806 (2002).
Soker, S., Takashima, S., Miao, H.Q., Neufeld, G. & Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735–745 (1998).
Gale, N.W. et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role Is rescued by angiopoietin-1. Dev. Cell 3, 411–423 (2002).
Carmeliet, P. et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 (1996).
Ferrara, N. et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442 (1996).
Bellomo, D. et al. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ. Res. 86, E29–E35 (2000).
Aase, K. et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation 104, 358–364 (2001).
Carmeliet, P. et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 7, 575–583 (2001).
Puri, M.C., Rossant, J., Alitalo, K., Bernstein, A. & Partanen, J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 14, 5884–5891 (1995).
Kubo, H. et al. Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood 96, 546–553 (2000).
Laakkonen, P., Porkka, K., Hoffman, J.A. & Ruoslahti, E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med. 8, 751–755 (2002).
Sainio, K. Experimental methods for studying urogenital development. The Kidney. From normal development to congenital disease (eds. Vize, P.D., Woolf, A.S. & Bard, J.B.L.) 327–342 (Elsevier Science, San Diego, 2003).
Sainio, K. et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development 124, 4077–4087 (1997).
Larsen, W.J. Human Embryology. 192 (Harcourt, New York, 1993).
Acknowledgements
We thank K. Karila and E. Valdre for their help in generating mice; S. Nuutinen and M. Jauhiainen for analysis of chylous fluid; H. Kubo, E. Ruoslahti, M. Quintanilla, H. Kowalski and D. Kerjaschki for antibodies; and T. Tainola, S. Karttunen, K. Makkonen, A. Parsons, P. Hyvarinen, M. Pajuportti, A. Flint and A. Vihera for technical assistance. Supported by grants from the Finnish Cancer Organizations, Academy of Finland (202852 and 204312), Novo Nordisk Foundation, Helsinki University Hospital Funds (TYH2301 and TYH1313), The Human Frontier Science Foundation, the European Union (Angionet QLC1-CT-2001-01172) and National Institutes of Health (HD37243).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Karkkainen, M., Haiko, P., Sainio, K. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5, 74–80 (2004). https://doi.org/10.1038/ni1013
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1013
This article is cited by
-
The cytoskeleton adaptor protein Sorbs1 controls the development of lymphatic and venous vessels in zebrafish
BMC Biology (2024)
-
Copper nanoparticles and silver nanoparticles impair lymphangiogenesis in zebrafish
Cell Communication and Signaling (2024)
-
Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
Toward Characterizing Lymphatic Vasculature in the Mammary Gland During Normal Development and Tumor-Associated Remodeling
Journal of Mammary Gland Biology and Neoplasia (2024)
-
The role of CD146 in renal disease: from experimental nephropathy to clinics
Journal of Molecular Medicine (2024)