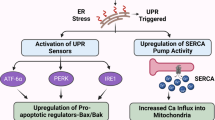
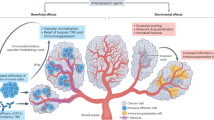
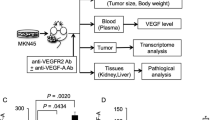
Abstract
Antiangiogenesis agents that target the VEGF/VEGF receptor pathway have become an important part of standard therapy in multiple cancer indications. With expanded clinical experience with this class of agents has come the increasing recognition of the diverse adverse effects related to disturbance of VEGF-dependent physiological functions and homeostasis in the cardiovascular and renal systems, as well as wound healing and tissue repair. Although most adverse effects of VEGF inhibitors are modest and manageable, some are associated with serious and life-threatening consequences, particularly in high-risk patients and in certain clinical settings. This Review examines the toxicity profiles of anti-VEGF antibodies and small-molecule inhibitors. The potential mechanisms of the adverse effects, risk factors, and the implications for selection of patients and management are discussed.
Key Points
-
Adverse effects associated with both VEGF- and VEGFR-targeting monoclonal antibodies and tyrosine kinase inhibitors are diverse, and include hypertension, arterial thromboembolic events, proteinuria, bowel perforation, reversible posterior leukoencephalopathy syndrome, wound complications and hemorrhage
-
Risk of serious adverse events may be increased by a multitude of risk factors related to the tumor characteristics and locations, comorbidities, and prior or concurrent anticancer therapy
-
Risk–benefit assessment is important for individual patients considering antiangiogenesis therapy
-
In order to provide evidence-based guidance for risk identification, toxicity management and treatment adjustment for antiangiogenesis agents further research in this area is warranted
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ferrara, N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin. Oncol. 29, 10–14 (2002).
Sane, D. C., Anton, L. & Brosnihan, K. B. Angiogenic growth factors and hypertension. Angiogenesis 7, 193–201 (2004).
van Heeckeren, W. J., Ortiz, J., Cooney, M. M. & Remick, S. C. Hypertension, proteinuria, and antagonism of vascular endothelial growth factor signaling: clinical toxicity, therapeutic target, or novel biomarker? J. Clin. Oncol. 25, 2993–2995 (2007).
Hood, J. D., Meininger, C. J., Ziche, M. & Granger, H. J. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am. J. Physiol. 274, H1054–H1058 (1998).
Horowitz, J. R. et al. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a maintenance role in quiescent adult endothelium. Arterioscler. Thromb. Vasc. Biol. 17, 2793–2800 (1997).
Ciuffetti, G. et al. Capillary rarefaction and abnormal cardiovascular reactivity in hypertension. J. Hypertens. 21, 2297–2303 (2003).
Steeghs, N. et al. VEGFR2 blockade in patients with solid tumors: mechanisms of hypertension and effects on vascular function [abstract]. J. Clin. Oncol. 24, A3037 (2006).
Yang, J. C. et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 349, 427–434 (2003).
Maitland, M. L. et al. Blood pressure (BP) as a biomarker for sorafenib (S.), an inhibitor of the vascular endothelial growth factor (VEGF) signaling pathway [abstract]. ASCO Meeting Abstracts. 24, 2035 (2006).
Faivre, S. et al. Safety, pharmacokinetic, and antitumor activity of su11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J. Clin. Oncol. 24, 25–35 (2006).
Furuse, J. et al. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 99, 159–165 (2008).
Minami, H. et al. Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors. Cancer Sci. 99, 1492–1498 (2008).
Drevs, J. et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 25, 3045–3054 (2007).
Wedge, S. R. et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 65, 4389–4400 (2005).
Choueiri, T. K. Axitinib, a novel anti-angiogenic drug with promising activity in various solid tumors. Curr. Opin. Investig. Drugs 9, 658–671 (2008).
Hu-Lowe, D. D. et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin. Cancer Res. 14, 7272–7283 (2008).
Wilhelm, S. M. et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109 (2004).
Escudier, B. et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356, 125–134 (2007).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Demetri, G. D. et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368, 1329–1338 (2006).
Motzer, R. J. et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 356, 115–124 (2007).
Schneider, B. P. et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J. Clin. Oncol. 26, 4672–4678 (2008).
Chobanian, A. V. et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289, 2560–2572 (2003).
Avastin® (bevacizumab) package insert (Genentech Inc., 2008).
Eppler, S. M. et al. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin. Pharmacol. Ther. 72, 20–32 (2002).
Scappaticci, F. A. et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J. Natl Cancer Inst. 99, 1232–1239 (2007).
Roncalli, J. et al. Bevacizumab in metastatic colorectal cancer: a left intracardiac thrombotic event. Ann. Oncol. 17, 1177–1178 (2006).
Giordano, F. J. et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc. Natl Acad. Sci. USA 98, 5780–5785 (2001).
Sarzani, R., Arnaldi, G. & Chobanian, A. V. Hypertension-induced changes of platelet-derived growth factor receptor expression in rat aorta and heart. Hypertension 17, 888–895 (1991).
Liu, J., Wu, L. L., Li, L., Zhang, L. & Song, Z. E. Growth-promoting effect of platelet-derived growth factor on rat cardiac myocytes. Regul. Pept. 127, 11–18 (2005).
Sutent® (sunitinib) package insert (Pfizer Labs, 2006).
Miller, K. D. et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J. Clin. Oncol. 23, 792–799 (2005).
Karp, J. E. et al. Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: therapy with sequential 1-{beta}-d-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin. Cancer Res. 10, 3577–3585 (2004).
D'Adamo, D. R. et al. Phase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomas. J. Clin. Oncol. 23, 7135–7142 (2005).
Miller, K., O'Neill, A., Perez, E., Seidman, A. & Sledge, G. W. Phase II feasibility trial incorporating bevacizumab into dose-dense doxorubicin and cyclophosphamide followed by paclitaxel in patients with lymph node-positive breast cancer: a trial of the Eastern Cooperative Oncology Group (E2104) [abstract]. ASCO Meeting Abstracts 26, 520 (2008).
Chu, T. F. et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370, 2011–2019 (2007).
Khakoo, A. Y. et al. Heart failure associated with sunitinib malate. Cancer 112, 2500–2508 (2008).
Goodman, V. L. et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin. Cancer Res. 13, 1367–1373 (2007).
Eremina, V. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111, 707–716 (2003).
Schrijvers, B. F., Flyvbjerg, A. & De Vriese, A. S. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 65, 2003–2017 (2004).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Barakat, R. K. et al. Interstitial nephritis secondary to bevacizumab treatment in metastatic leiomyosarcoma. Ann. Pharmacother. 41, 707–10 (2007).
Frangié, C. et al. Renal thrombotic microangiopathy caused by anti-VEGF-antibody treatment for metastatic renal-cell carcinoma. Lancet Oncol. 8, 177–178 (2007).
Roncone, D., Satoskar, A., Nadasdy, T., Monk, J. P. & Rovin, B. H. Proteinuria in a patient receiving anti-VEGF therapy for metastatic renal cell carcinoma. Nat. Clin. Pract. Nephrol. 3, 287–293 (2007).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Miller, K. et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 357, 2666–2676 (2007).
Giantonio, B. J. et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 25, 1539–1544 (2007).
Sandler, A. et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 355, 2542–2550 (2006).
Escudier, B. et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370, 2103–2111 (2007).
Rixe, O. et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 8, 975–984 (2007).
Izzedine, H., Brocheriou, I., Deray, G. & Rixe, O. Thrombotic microangiopathy and anti-VEGF agents. Nephrol. Dial. Transplant. 22, 1481–1482 (2007).
Bollée, G. et al. Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol. Dial. Transplant. 24, 682–685 (2009).
Kapiteijn, E., Brand, A., Kroep, J. & Gelderblom, H. Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome. Ann. Oncol. 18, 1745–1747 (2007).
Levey, S. A. et al. Thrombotic microangiopathy associated with sunitinib, a VEGF inhibitor, in a patient with factor V Leiden mutation. NDT Plus 1, 154–156 (2008).
Feldman, D. et al. Phase I trial of bevacizumab plus sunitinib in patients with metastatic renal cell carcinoma [abstract]. ASCO Meeting Abstracts 26, 5100 (2008).
Spano, J. P. et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet 371, 2101–2108 (2008).
Isambert, N. et al. A phase I dose escalation and pharmacokinetic (PK) study of intravenous aflibercept (VEGF trap) plus docetaxel (D) in patients (pts) with advanced solid tumors: Preliminary results [abstract]. ASCO Meeting Abstracts 26, 3599 (2008).
Novotny, W. et al. Identification of squamous cell histology and central, cavitary tumors as possible risk factors for pulmonary hemorrhage (PH) in patients with advanced NSCLC receiving Bevacizumab (BV) [abstract]. Proc. Am. Soc. Clin. Oncol. 20, 1318 (2001).
Hanna, N., von Pawel, J., Reck, M. & Scagliotti, G. Carboplatin/paclitaxel with/without sorafenib in chemonaive patients with stage IIIB-IV non-small cell lung cancer (NSCLC): Interim analysis (IA) results from a randomized phase III trial (ESCAPE) [abstract]. J. Thorac. Oncol. 3, A13 (2008).
Socinski, M. A. et al. Efficacy and safety of sunitinib in previously treated, advanced non-small cell lung cancer (NSCLC): Preliminary results of a multicenter phase II trail [abstract]. ASCO Meeting Abstracts 24, 7001 (2006).
Amgen Press Release. Amgen, Takeda and Millennium provide update on phase 3 trial of Motesanib in patients with non-small cell lung cancer. http://www.amgen.com/media/media_pr_detail.jsp?year=2008&releaseID=1228588 (2008).
Sandler, A. et al. Retrospective study of clinical and radiographic risk factors associated with early onset, severe pulmonary hemorrhage in bevacizumab-treated patients with advanced non-small cell lung cancer (NSCLC) [abstract]. ASCO Meeting Abstracts 26, 8074 (2008).
McDermott, D. F. et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J. Clin. Oncol. 26, 2178–2185 (2008).
Burger, R. A., Sill, M. W., Monk, B. J., Greer, B. E. & Sorosky, J. I. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 25, 5165–5171 (2007).
Cannistra, S. A. et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J. Clin. Oncol. 25, 5180–5186 (2007).
Han, E. S. & Monk, B. J. Bevacizumab in the treatment of ovarian cancer. Expert Rev. Anticancer Ther. 7, 1339–1345 (2007).
Nexavar® (sorafenib) package insert (Bayer HealthCare Pharmaceuticals Inc., 2008).
Streit, M. et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 19, 3272–3282 (2000).
Scappaticci, F. A. et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J. Surg. Oncol. 91, 173–180 (2005).
Allegra, C. J. et al. Initial safety report of NSABP C-08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J. Clin. Oncol. doi:10.1200/JCO.2009.21.9220.
ClinicalTrial.gov, [online] (2009).
Lu, J. F. et al. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother. Pharmacol. 62, 779–786 (2008).
Wedam, S. B. et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J. Clin. Oncol. 24, 769–777 (2006).
Stott, V. L., Hurrell, M. A. & Anderson, T. J. Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Intern. Med. J. 35, 83–90 (2005).
Lamy, C., Oppenheim, C., Méder, J. F. & Mas, J. L. Neuroimaging in posterior reversible encephalopathy syndrome. J. Neuroimaging 14, 89–96 (2004).
Glusker, P., Recht, L. & Lane, B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N. Engl. J. Med. 354, 980–982 (2006).
Allen, J. A., Adlakha, A. & Bergethon, P. R. Reversible posterior leukoencephalopathy syndrome after bevacizumab/folfiri regimen for metastatic colon cancer. Arch. Neurol. 63, 1475–1478 (2006).
Govindarajan, R., Adusumilli, J., Baxter, D. L., El-Khoueiry, A. & Harik, S. I. Reversible posterior leukoencephalopathy syndrome induced by RAF kinase inhibitor BAY 43–9006 J. Clin. Oncol. 24, e48 (2006).
Martín, G., Bellido, L. & Cruz, J. J. Reversible posterior leukoencephalopathy syndrome induced by sunitinib. J. Clin. Oncol. 25, 3559 (2007).
Marinella, M. A. & Markert, R. J. Reversible posterior leukoencephalopathy syndrome associated with anticancer drugs. Intern. Med. J. doi:10.1111/j.1445–59942008.01829.x.
Katoh, O., Tauchi, H., Kawaishi, K., Kimura, A. & Satow, Y. Expression of the vascular endothelial growth factor (VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory effect of vegf on apoptotic cell death caused by ionizing radiation. Cancer Res. 55, 5687–5692 (1995).
Gerber, H. P. et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417, 954–958 (2002).
Nalluri, S. R., Chu, D., Keresztes, R., Zhu, X. & Wu, S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 300, 2277–2285 (2008).
Kamba, T. et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 290, H560–H576 (2006).
Desai, J. et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann. Intern. Med. 145, 660–664 (2006).
Ramalingam, S. S. et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J. Clin. Oncol. 26, 60–65 (2008).
Azad, N. S. et al. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin. Cancer Res. 15, 1411–1416 (2009).
Wakelee, H. et al. Cooperative group research efforts in lung cancer 2008: focus on advanced-stage non-small-cell lung cancer. Clin. Lung Cancer 9, 346–351 (2008).
Azad, N. S. et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J. Clin. Oncol. 26, 3709–3714 (2008).
Puzanov, I. et al. Final results of a phase I trial of sorafenib and bevacizumab in patients with metastatic renal cell cancer (mRCC) [abstract]. AACR Meeting Abstracts 2007, A19 (2007).
Merchan, J. R. et al. Phase I/II trial of CCI-779 and bevacizumab in stage IV renal cell carcinoma: Phase I safety and activity results [abstract]. ASCO Meeting Abstracts 25, 5034 (2007).
Zafar, Y. et al. Preliminary results of a phase I study of bevacizumab (BV) in combination with everolimus (E) in patients with advanced solid tumors [abstract]. ASCO Meeting Abstracts 24, 3097 (2006).
Saltz, L. B. et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 26, 2013–2019 (2008).
Miles, D. et al. Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO [abstract]. ASCO Meeting Abstracts 26, LBA1011 (2008).
Manegold, C. et al. Randomised, double-blind multicentre phase III study of bevacizumab in combination with cisplatin and gemcitabine in chemotherapy-naive patients with advanced or recurrent non-squamous non-small cell lung cancer (NSCLC): BO17704 [abstract]. ASCO Meeting Abstracts 25, LBA7514 (2007).
Sridhar, S. S. et al. Activity of cediranib (AZD2171) in patients (pts) with previously untreated metastatic renal cell cancer (RCC). A phase II trial of the PMH Consortium [abstract]. ASCO Meeting Abstracts 26, 5047 (2008).
Hutson, T. E. et al. Biomarker analysis and final efficacy and safety results of a phase II renal cell carcinoma trial with pazopanib (GW786034), a multi-kinase angiogenesis inhibitor [abstract]. ASCO Meeting Abstracts 26, 5046 (2008).
Grothey, A. et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J. Clin. Oncol. 26, 5326–5334 (2008).
Garcia, A. A. et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J. Clin. Oncol. 26, 76–82 (2008).
Wright, J. D. et al. Bevacizumab combination therapy in recurrent, platinum-refractory, epithelial ovarian carcinoma: a retrospective analysis. Cancer 107, 83–89 (2006).
Agarwala, S. S. et al. Randomized phase III study of paclitaxel plus carboplatin with or without sorafenib as second-line treatment in patients with advanced melanoma [abstract]. ASCO Meeting Abstracts 25, 8510 (2007).
Acknowledgements
The authors wish to thank Drs. H. Streicher and S. P. Ivy for critical review and comments, and Mr. C. Risch for assistance in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chen, H., Cleck, J. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol 6, 465–477 (2009). https://doi.org/10.1038/nrclinonc.2009.94
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.94
This article is cited by
-
Novel heavily fucosylated glycans as a promising therapeutic target in colorectal cancer
Journal of Translational Medicine (2023)
-
Angiogenic signaling pathways and anti-angiogenic therapy for cancer
Signal Transduction and Targeted Therapy (2023)
-
Newly synthesized methionine aminopeptidase 2 inhibitor hinders tumor growth
Drug Delivery and Translational Research (2023)
-
Long-Term Change in Renal Function After Intravitreal Anti-VEGF Treatment for Diabetic Macular Edema: A 2-Year Retrospective Cohort Study
Ophthalmology and Therapy (2023)
-
Intricate role of sleep deprivation in modulating depression: focusing on BDNF, VEGF, serotonin, cortisol, and TNF-α
Metabolic Brain Disease (2023)