Article Text
Abstract
Introduction Breathlessness is a cardinal symptom in cardiorespiratory disease. An instrument for measuring different aspects of breathlessness was recently developed, the Multidimensional Dyspnea Profile (MDP). This study aimed to validate the MDP in terms of the underlying factor structure, internal consistency, test–retest reliability and concurrent validity in Swedish outpatients with cardiorespiratory disease.
Methods Outpatients with stable cardiorespiratory disease and breathlessness in daily life were recruited. Factor structure of MDP was analysed using confirmatory factor analysis; internal consistency was analysed using Cronbach’s alpha; and test–retest reliability was analysed using intraclass correlation coefficients (ICCs) for patients with unchanged breathlessness between assessments (baseline, after 30–90 min and 2 weeks). Concurrent validity was evaluated using correlations with validated scales of breathlessness, anxiety, depression and health-related quality of life.
Results In total, 182 outpatients with cardiorespiratory disease and breathlessness in daily life were included; 53.3% were women; main diagnoses were chronic obstructive pulmonary disease (24.7%), asthma (21.4%), heart failure (19.2%) and idiopathic pulmonary fibrosis (18.7%). The MDP total, immediate perception and emotional response scores, and individual item scores showed expected factor structure and acceptable measurement properties: internal consistency (Cronbach’s alpha, range 0.80–0.93); test–retest reliability at 30–90 min and 2 weeks (ICC, range 0.67–0.91); and concurrent validity. There was no evidence of a learning effect. Findings were similar between diagnoses.
Discussion MDP is a valid instrument for multidimensional measurement of breathlessness in Swedish outpatients across cardiorespiratory diseases.
- dyspnoea
- breathlessness
- multidimensional
- respiratory disease
- heart disease
- measurement
- Swedish
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
This observational cohort study evaluated whether the Multidimensional Dyspnea Profile (MDP) is reliable valid for measuring breathlessness in Swedish outpatients with cardiorespiratory disease.
MDP showed good test properties for measuring different dimensions of breathlessness.
This validation (N=182) provides novel data on test–retest reliability at 30-90 min and 2 weeks, and the lack of evidence for a learning effect, and findings were similar between chronic obstructive pulmonary disease and other conditions.
Introduction
Breathlessness, the feeling of breathing discomfort, is a cardinal symptom of cardiorespiratory diseases and is strongly associated with adverse health outcomes.1 Breathlessness is linked to reduced physical activity, worsening deconditioning, increased anxiety and depression, impaired quality of life, increased risk of hospitalisation and earlier death.1 2 Breathlessness is associated with worse prognosis across cardiorespiratory diseases3 4 and is a stronger predictor of mortality than the level of airflow limitation in chronic obstructive pulmonary disease (COPD).3 In patients with suspected heart disease undergoing cardiac stress testing, more severe breathlessness is a strong risk factor for earlier death overall and from cardiac disease.5
Breathlessness consists of several different qualitatively distinct sensations that vary in intensity.1 Several dimensions of this symptom can be differentiated by the individual: the experienced intensity and unpleasantness, the associated emotional response and the functional impact on the person’s life.1
Despite its serious impact, breathlessness remains frequently under-reported, unmeasured and insufficiently treated in clinical practice.6 The level of unpleasantness, emotional responses and how the breathlessness feels (sensory qualities (SQs)) have been measured in different studies using different, often disease-specific instruments with varying types of scales, wordings and time frames.1 7 This makes it difficult to compare findings between studies, patient populations and settings. Standardised multidimensional measurement is essential to adequately capture treatment effects in clinical trials as different treatments may target different dimensions of breathlessness.8 9 Opioids have been found to have a stronger effect on the experienced unpleasantness and associated anxiety from breathlessness than on the intensity.8 Patients with COPD with a high level of anxiety and breathlessness-related fear benefit from pulmonary rehabilitation,10 which positively affects the patient’s coping and function in relation to breathlessness rather than the intensity of the symptom.9
The Multidimensional Dyspnea Profile (MDP) is a recently developed tool to separately measure the immediate unpleasantness or discomfort of breathing (A1 domain), presence and intensity of five SQs, and intensity of five emotional responses of breathlessness.7 The MDP was developed to measure breathlessness across underlying diseases and settings (laboratory and non-laboratory).11 12 The MDP builds on extensive mechanistic studies of multidimensional pain and breathlessness.7 The MDP was first tested in response to laboratory stimuli7 and then validated in 151 patients admitted to an emergency department for acute breathlessness (29% had asthma, 27% had COPD, 19% had pneumonia, 13% had heart failure, and 13% had other conditions).11 The MDP has been translated and used in several languages, including French (language-specific versions for France, Belgium and Canada), German, Dutch (language-specific versions for Belgium and the Netherlands), English (language specific versions for Canada and the UK) and Swedish.7 13 14
The MDP can either be administered by an investigator/healthcare provider or be self-completed with a person on hand to answer questions during initial administration.7 The time frame or situation of the measurement is defined by the user.7 Before use, it is important that the respondent receives standardised information and instructions as described elsewhere,7 for a reliable and valid measurement. The MDP consists of 11 items divided into three domains.7 In the first domain, the unpleasantness or discomfort of the breathing sensation is rated on a numerical rating scale (NRS) between 0 (‘neutral’) and 10 (‘unbearable’). In the second domain, the respondent first indicates which of the five descriptions matches their breathing discomfort and indicates the most accurate descriptor. The respondent then rates the intensity of each descriptor (and of another self-specified sensation if needed) on an NRS between 0 (‘none’) and 10 (‘as intense as I can imagine’). In the third domain, the respondent rates the intensity of emotional responses to their breathing discomfort (depression, anxiety, frustration, anger and fright) on an NRS between 0 (‘none’) and 10 (‘the most I can imagine’).7
Validation studies in outpatients have been performed in Australia15 and in France,13 but only for patients with COPD. There is currently no validated instrument for multidimensional measurement of breathlessness in Swedish and no published validation of MDP in outpatients across different cardiorespiratory diseases.
The primary aim was therefore to validate the Swedish translation of MDP in terms of the underlying factor structure, internal consistency, test–retest reliability and concurrent validity in outpatients with cardiorespiratory disease. The secondary aim was to compare the measurement properties between patients with COPD and patients with other cardiorespiratory diagnoses.
Methods
Design and population
This was a prospective, multicenter cohort study of Swedish stable outpatients with breathlessness and diagnosed cardiorespiratory disease in accordance with current guidelines.16–19 Patients were recruited at routine appointments at five outpatient clinics (Karlskrona; Karolinska University Hospital, Solna; Umeå; Uppsala and Örebro). The database was used for a validation study of the Swedish version of Dyspnea-12 questionnaire.20 These validations were published separately as prespecified in the project protocol due to the number of analyses involved and the difference in items between the instruments.
Inclusion criteria were age 18 years or older, documented physician-diagnosed chronic respiratory and/or cardiac disease, self-reported breathlessness during daily life defined as an answer ‘yes’ to the question ‘Did you experience any breathlessness during the last 2 weeks?’ and ability to give written informed consent to participate in the study. Exclusion criteria were inability to write or understand Swedish adequately to participate, cognitive or other inability to participate in the study, and estimated survival of less than 3 months.
Patient and public involvement
The design of the original MDP involved input from patients, including the categorisation of the descriptors of their breathlessness. There was no specific patient or public involvement in the design or conduct of the present validation study. The findings will be disseminated through posters in the clinic and through newspapers.
Assessments
Data were collected at the first clinical visit (baseline), including a repeat questionnaire after 30–90 min and by a postal questionnaire after 2 weeks.
At baseline, the patient completed a questionnaire on demographics, smoking status and pack-years of smoking, the published linguistically validated Swedish version of MDP.14 The MDP was used in its intended format, including the standardised written instructions rating intensity and unpleasantness of the respiratory sensations.14 The time period used was ‘during the last 2 weeks’. Other assessments included severity of breathlessness (0–10 NRS); severity of breathlessness on average during the last 2 weeks (Likert scale); the modified Medical Research Council Breathlessness Scale (mMRC); health status using the Chronic Obstructive Pulmonary Disease Assessment Test (CAT), which has been validated in COPD and interstitial lung disease,21 22 and the generic instrument EuroQol Five Dimensions–Five Levels (EQ-5D-5L) (higher values indicating better health status)23; the Hospital Anxiety and Depression Scale (HADS)24; fatigue (Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue Scale); and average severity of pain (0–10 NRS). Except for current distressing breathlessness, the time period for all self-reported measures was ‘during the last 2 weeks’.
Clinical data were obtained from the patient’s medical records by the responsible clinician on diagnosed disease, current medications, height and weight, spirometry prebronchodilator and/or postbronchodilator values of forced expiratory volume in one second (FEV1) and forced vital capacity. Spirometry values from the last 12 months were accepted if deemed representative of the patient’s current condition, and postbronchodilation values were used if available.
At 30–120 min after completing the first questionnaire, patients again self-rated MDP and change in breathlessness since the first assessment on a 7-point ordinal scale (the Global Impression of Change (GIC); 1=‘very much better’, 4=‘no change’ and 7 = ‘very much worse’). The postal questionnaire included repeated measurement of MDP and GIC. The MDP was used in the Swedish linguistically validated version.14 The translation and linguistic validation were performed by specialists in the field (Mapi SAS, Language Services Unit, Lyon, France) in accordance with international guidelines for patient-reported outcomes for interventional trials.25 26
Statistical analyses
Baseline characteristics were tabulated using descriptive statistics. The measurement properties of the MDP were evaluated for the total score (sum of A1 unpleasantness score and intensities for the five SQs and five emotional responses; range 0–110), the immediate perception subdomain score (sum of the A1 score and five SQ intensities, range 0–60), emotional response subdomain score (sum of intensities for the five emotional responses, range 0–50)7 11 14 and the immediate unpleasantness of breathlessness (A1 domain, 0–10 NRS). Summary scores, including MDP, CAT and HADS, were set to missing if data on any of the subitems of the score were unavailable, to avoid the influence of missing items on the comparisons. No data were imputed.
The factor structure of MDP was analysed using confirmatory factor analysis. Model fit was assessed using the root mean square error of approximation (RMSEA)27 and Bentler’s comparative fit index.28 Fit and factor loadings were compared with previous comparable validation studies that used similar methodology.11 12 15
Internal consistency was analysed using Cronbach’s alpha. Test–retest reliability was analysed using intraclass correlation coefficients using two-way mixed analysis of variance for patients who reported their breathlessness to be unchanged (GIC score=4) at follow-up assessments after 30–90 min and after 2 weeks, respectively. The recall period of 2 weeks was chosen as previous data indicate that the test–retest reliability of the MDP can be weak for recall after 4 weeks.12 A potential learning effect (change in MDP scoring related to familiarisation with the questionnaire) was evaluated by comparing the test–retest reliability at 2 weeks compared with (1) the first assessment and (2) second assessment (after 30–90 min). Test–retest reliability and agreement were evaluated only in patients who reported that their breathlessness was unchanged on the GIC at both follow-up time points. Agreement was evaluated using a Bland-Altman plot. Concurrent validity was evaluated using Pearson’s correlation coefficient of the baseline MDP overall, immediate perception and emotional response scores with FEV1; mMRC; CAT; EQ-5D-5L; HADS total, anxiety and depression scores; and the FACIT-Fatigue Scale.
All analyses were conducted for all participants and for people with COPD and other diagnoses as main cause of breathlessness, separately. Analyses were conducted with MATLAB R2018b (MathWorks, Natick, MA, USA). The prespecified sample size was 180 included patients based on previous validation studies.7 11 12 29 30
Results
A total of 182 patients were enrolled at five outpatient clinics between 29 August 2016 and 23 December 2017. The mean age was 68.6 (SD 13.8, range 19–91) years; 53.3% were women; and the main diagnoses were COPD (24.7%), asthma (21.4%), heart failure (19.2%) and idiopathic pulmonary fibrosis (IPF, 18.7%), as shown in table 1.
Baseline characteristics of 182 outpatients with cardiorespiratory disease
MDP scores and patient-reported outcomes at baseline are shown in table 2. Participants reported a mean breathlessness unpleasantness (A1 score) of 4.94 (SD 2.53), immediate perception score of 24.3 (SD 14.9) of maximum 60 and emotional response score of 16.0 (SD 13.5) of maximum 50. The most frequently selected SQs were breathing a lot and air hunger, which also had the highest mean intensities; and 81% of participants rated their breathlessness over the last 2 weeks as moderate to severe.
Patient-reported outcomes at baseline in 182 outpatients with cardiorespiratory disease
Follow-up data were available for 179 (98.4%) after 30–90 min and for 162 (89.0%) after 2 weeks. Baseline characteristics were similar for people with and without follow-up data (n=20) at 2 weeks. The actual time between the first visit and the subsequent follow-up (projected at 2 weeks) was a median of 14 days (IQR 14–18, mean 17.2, range 3–58).
Factor analysis was consistent with the previously proposed two-factor model of MDP (immediate perception and emotional response), although the fit of the two-factor model was not optimal (RMSEA=0.115 and Bentler’s comparative fit index=0.941). Factor loadings were similar to those of the original English version of MDP, with A1 unpleasantness and SQ intensities loading together (immediate perception domain), and the emotional response intensities loading together (emotional response domain); all loadings were >0.573 (see online supplementary table S1).
Supplemental material
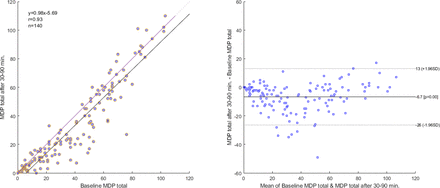
Internal consistency was moderate to high for the MDP total score (Cronbach’s alpha=0.933), the immediate perception (0.829) and the emotional response (0.798) domain. Test–retest reliability for the MDP total and subdomain scores and for individual items was moderate to high (table 3). The test–retest reliability was generally higher at 30–90 min than after 2 weeks as expected; was slightly lower for the A1 unpleasantness score than for the MDP total and subdomain scores; and was similar across the different SQ and emotional response intensities (table 3). As the test–retest reliability was similar overall for scores at 2 weeks when comparing with those from the first and second assessment, respectively; there was no clear evidence for any learning effect. Correlation, which was high at 0.93, and agreement for the MDP total score rated 30–90 min apart are shown in figure 1. The mean bias was −6.7 (95% CI −25 to 13). The difference between the first and the second assessments was largest for averages of MDP total between 20 and 50, where the trend was that the second assessment produced a lower total score than the first (figure 1).
Correlation and agreement of the MDP total score between baseline (Q1) and after 30–90 min (Q2) in 140 outpatients with cardiorespiratory disease who reported their breathlessness to be similar between the assessments on the GIC Scale (GIC score=4). Left panel: scatterplot with a 45 degree line (no difference) and a simple linear regression line with Pearson’s correlation coefficient (r). Right panel: Bland-Altman plot with lines for mean bias and 95% limits of agreement. GIC, Global Impression of Change; MDP, Multidimensional Dyspnea Profile.
Test–retest reliability for the Swedish MDP
Concurrent validity estimates for MDP total, immediate perception and emotional response, and A1 unpleasantness scores with patient-reported outcomes and FEV1 % of predicted are shown in figure 2. Higher MDP breathlessness intensity was most strongly associated with higher mMRC, CAT total, EQ-5D-5L total and more severe fatigue (lower FACIT-Fatigue scores). The pattern of correlations was fairly similar between the MDP scores, with the exception that the MDP affective score was more strongly correlated with higher HADS overall and anxiety scores compared with the MDP immediate perception and A1 unpleasantness scores (figure 2).
Concurrent validity for the Swedish MDP. Correlations for the MDP total, immediate perception, emotional response and A1 unpleasantness score with patient-reported outcomes and the FEV1 were analysed using Pearson’s correlation coefficient. The dotted lines correspond to an adjusted significance level of 0.002. anx, anxiety; CAT, Chronic Obstructive Pulmonary Disease Assessment Test; depr, depression; EQ-5D-5L, EuroQol Five Dimensions–Five Levels; FACIT, Functional Assessment of Chronic Illness Therapy; FEV1, forced expiratory volume in one second; HADS, Hospital Anxiety and Depression Scale; MDP, Multidimensional Dyspnea Profile; mMRC, Modified Medical Research Council Breathlessness Score; pred, predicted; tot, total.
The measurement properties of MDP were similar between patients with COPD (n=45) and patients with other diagnoses as the main cause of breathlessness (n=137), including the factor structure (see online supplementary table S1), internal consistency, test–retest reliability and concurrent validity (see online supplementary table S2).
Discussion
The main findings of this study are that in outpatients with cardiorespiratory disease, the Swedish version of MDP is valid in terms of the factor structure, test–retest reliability and concurrent validity for multidimensional measurement of breathlessness.
What this study adds
The present study is the first validation of MDP in outpatients across a range of important cardiorespiratory diseases. About 20% of included patients had COPD, asthma, heart failure and IPF, respectively, and the measurement properties were similar between people with COPD and with other diagnoses. The findings are consistent with previous studies of COPD outpatients only,13 15 as well as validation studies of the MDP developers in laboratory settings8 31 32 and patients with cardiorespiratory diseases in an acute emergency setting.7 11 12 The present study supports that MDP is valid for multidimensional measurement of breathlessness across settings and patient populations.
This is also the first clinical validation of a multidimensional instrument for breathlessness in Swedish. MDP showed expected measurement properties in terms of factor structure, internal consistency, test–retest reliability and concurrent validity, which are similar to those of previous validations in English from the USA7 11 12 and Australia,15 and in French.13 The present findings facilitate research of this important symptom across languages.
Novel data also include the association between MDP and fatigue (measured using FACIT-Fatigue Scale), which has not been previously reported.7 11–13 15 The moderate to strong association with fatigue supports the concurrent validity of the instrument and that MDP can be useful for exploring the important and still scarcely evaluated association between multidimensional breathlessness and fatigue.
The time period validated for breathlessness and patient-reported outcomes is during the past 2 weeks, which is consistent with the time period validated in Australia by Williams et al.15 The time period of measurement for MDP is to be specified by the user, depending on the setting and aim.7 Morélot-Panzini et al13 evaluated MDP in French for ‘the worst experience within the last 15 days’.13 The similar findings between studies indicate that, even if the actual intensity levels for items may vary, the measurement properties (ability) of MDP to measure multidimensional breathlessness are consistent across the different time periods.7 11–13 15 A novel finding is also that there was no systematic difference in the test–retest reliability between the first and the second assessments (as compared with that at 2 weeks), supporting that there was no evidence for a learning effect.
Comparison to the literature
The factor structure of the Swedish MDP was similar to that reported in previous studies. The fit of the two-factor model was not optimal but was similar or better than that in previous validations in the USA, Australia and France.11 13 15 The suboptimal fit can be interpreted as that there was no conclusive evidence that the proposed two-factor structure7 fitted the data significantly better than a model with more than two factors. Clustering was observed between A1 unpleasantness and the SQ intensities, and between the emotional response intensities, similarly to findings in previous validations.11 13 15 The associations with other patient-reported outcomes, with the exception of slight differences for HADS anxiety, were overall similar for the MDP immediate perception, emotional response and A1 unpleasantness scores. The two-factor structure, the independence of the subdomain scores and their response to changes in health status and to interventions should be further explored. While the internal consistency and the test–retest reliability were consistent with findings in previous validations,7 11 12 the present study also provided data on test–retest reliability after 30–90 min to evaluate the repeatability of the MDP. Importantly, the focal period in all analyses was breathlessness ‘the last 2 weeks’. Even if test–retest reliability was high for most subscores and items, there were some variability and differences seen (in the Bland-Altman plot of the MDP total score) between the ratings even 30–90 min apart that warrant further evaluation of the repeatability and minimal clinical important difference of the MDP. Finally, concurrent validity estimates in the present study were similar to those previously reported in other settings and languages.11 13 15
Strengths and limitations
The strengths of the present study are that it included a large sample of outpatients in clinical practice across a range of relevant cardiorespiratory diagnoses. Completeness of data was high for both baseline and follow-up at 2 weeks. Recommended standard methods, similar to those employed in previous studies, were used to facilitate comparison.
Limitations include that the number of patients in each of the non-COPD diagnosis groups was relatively small. Measurement properties for specific subpopulations could be detailed in further research. However, the present analysis shows no evidence that the measurement properties of MDP differ substantially between outpatients with COPD and other cardiorespiratory diagnoses. The findings pertain to patients who were able to read and understand Swedish. The study was conducted in the setting of clinical outpatient clinics, which might affect the standardisation of the conditions, including the information and instructions given in relation to the assessments. At the clinical visit, the staff was instructed to inform the participants that the questions mostly pertained to experiences during the past 2 weeks. The participants were asked to read the instructions of the questionnaires carefully and could ask questions as needed. For feasibility, the participants were instructed to complete and return the follow-up questionnaire at 2 weeks by post. We think that these procedures increased feasibility and completeness of data collection and that the close connection to clinical care increases the external validity of the findings.
Implications
For research and clinical care, a first instrument—the MDP—for multidimensional assessment of breathlessness is now available in Swedish. The measurement properties are similar to those shown in English and French, and for COPD outpatients and patients with breathlessness in the acute emergency setting. These findings support that MDP is valid for measurement and comparisons of breathlessness dimensions across disease populations, settings and languages. The intention and an advantage of the MDP is that items or sets of items can be used (instead of using the whole instrument) as needed, depending on the aim of the measurement.7 Standardised instructions and conditions are essential for reliable and valid measurements.7 The lack of a learning effect in the present study supports that repeating the test at each time point (for familiarisation) is not needed. This increases the feasibility of the instrument.
The MDP can be used free of charge in the context of not-funded academic research, and distribution fee will apply in the context of funded academic and commercial use.7 The MDP is distributed by the Mapi Research Trust (https://eprovide.mapi-trust.org), which should be contacted for any enquiry about the questionnaire and the requirements regarding its use.
Further research should validate MDP in people with breathlessness and other conditions than cardiorespiratory disease. Other topics include a potential learning effect in successive scoring and to further validate the factor structure, relationship and independence between different MDP items and aspects of breathlessness. Standardised multidimensional symptom measurement using MDP could be of fundamental importance for improved research and clinical care of patients suffering from breathlessness.
Acknowledgments
The authors thank all research nurses who dedicated their time and work to conduct this study: nurses at the respiratory outpatient clinic in Karlskrona; Lisa Carlson, Karolinska University Hospital Solna, Stockholm; Annika Johansson and Frida Holmström, University Hospital, Umeå; Karin Johansson, Örebro University Hospital; and Jonatan Blomqvist, Lund, for help with data input and quality checking. The authors extend their warm thanks to all patients who made this research possible.
References
Footnotes
Contributors Conception, design and first draft: ME; data collection: ME, MS, CJ, AB, JaS, HI and JoS; statistical analysis: HB and AB-H; interpretation, revision for important intellectual content and approval of the version to be published: all authors.
Funding The translation was funded by unrestricted grants from the Swedish Respiratory Society, the Swedish Heart−Lung Foundation and the Swedish Society for Medical Research.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Written informed consent was obtained from all participants. The protocol was approved by the regional ethical review board at Lund University (DNr: 2016/16). The Multidimensional Dyspnea Profile was used in this project with the permission of the copyright holder, Professor Robert B. Banzett, USA.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.