Article Text
Abstract
The Faculty of Intensive Care Medicine and Intensive Care Society Guideline Development Group have used GRADE methodology to make the following recommendations for the management of adult patients with acute respiratory distress syndrome (ARDS). The British Thoracic Society supports the recommendations in this guideline. Where mechanical ventilation is required, the use of low tidal volumes (<6 ml/kg ideal body weight) and airway pressures (plateau pressure <30 cmH2O) was recommended. For patients with moderate/severe ARDS (PF ratio<20 kPa), prone positioning was recommended for at least 12 hours per day. By contrast, high frequency oscillation was not recommended and it was suggested that inhaled nitric oxide is not used. The use of a conservative fluid management strategy was suggested for all patients, whereas mechanical ventilation with high positive end-expiratory pressure and the use of the neuromuscular blocking agent cisatracurium for 48 hours was suggested for patients with ARDS with ratio of arterial oxygen partial pressure to fractional inspired oxygen (PF) ratios less than or equal to 27 and 20 kPa, respectively. Extracorporeal membrane oxygenation was suggested as an adjunct to protective mechanical ventilation for patients with very severe ARDS. In the absence of adequate evidence, research recommendations were made for the use of corticosteroids and extracorporeal carbon dioxide removal.
- ARDS
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Aims
The purpose of this guideline is to provide an evidence-based framework for the management of adult patients with acute respiratory distress syndrome (ARDS) that will inform both key decisions in the care of individual patients and broader policy. Our recommendations are neither dictates nor standards of care. We cannot take into account all of the features of individual patients and complex local factors; all we can do is to synthesise relevant evidence and to put it into the context of current critical care medicine. Similarly, our recommendations are not comprehensive: these guidelines have relevance to a fraction of the total number of decisions that are required of carers for these complex patients. Indeed, the current state of the art for the management of ARDS has been recently reviewed1–4 and comparable guidelines have been produced by national and international stakeholders.5 6
Scope
The topics considered were chosen by the Guideline Development Group (GDG) in the light of results from a survey carried out for the Intensive Care Society (ICS), including 556 responses from 3200 members. Popular topics were excluded by the GDG if it was felt that there was a dearth of evidence (eg, appropriate diagnostic investigations and the role of specialist centres), when the evidence was not specific to ARDS (weaning from mechanical ventilation, nutrition and the timing of tracheostomy) and if there was overlap with existing guidelines (post-ICU (intensive care unit) care and rehabilitation).
Definitions
ARDS was first reported in a case series from Denver in 1967.7 The American European Consensus Conference (AECC) 1994 defined ARDS as ‘an acute inflammatory syndrome manifesting as diffuse pulmonary oedema and respiratory failure that cannot be explained by, but may co-exist with, left-sided heart failure’.8 In 2012, the AECC definition was re-evaluated and minor alterations were proposed by the European Society of Intensive Care Medicine ARDS Definition Task Force. This iteration recognised 3 grades of severity depending on the degree of hypoxaemia and stipulated the application of at least 5 cmH2O of positive end-expiratory pressure (PEEP) or continuous positive airway pressure. This so-called Berlin definition was validated using retrospective cohorts and captures patients with a mortality of 24% in patients with mild ARDS, rising to 48% in the group of patients with the most severe respiratory failure.9
A four-point lung injury scoring system (Murray Score or LIS) is the most widely used means of quantifying ARDS severity. It is based on the level of PEEP, the ratio of the partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2), the dynamic lung compliance and the degree of radiographic infiltration.7 Although the LIS has been widely used in clinical studies and a score of >3.0 is commonly used as a qualifying threshold for support with extracorporeal membrane oxygenation (ECMO), it cannot predict outcome during the first 24–72 hours of ARDS.8 When the scoring system is used 4–7 days after the onset of the syndrome, scores of 2.5 or higher predicted a complicated course requiring prolonged mechanical ventilation.9
As a syndrome rather than a disease, there is no laboratory, imaging or other ‘gold standard’ diagnostic investigation for ARDS. Therefore like acute kidney injury (AKI), ARDS is caused by a huge range of conditions and as a consequence patients with ARDS are heterogeneous. The outcome of these patients is determined by the underlying causes of ARDS, patient-specific factors such as comorbidities, clinical management and the severity of illness.
Epidemiology and outcomes
Using the AECC definition, several population-based studies of ARDS showed a fairly consistent picture of the age, mortality and severity of illness; however, there was almost a fourfold difference in incidence, probably contributed to by differences in study design and ICU utilisation.10 In the USA, there are estimated to be 190 000 cases and 74 000 deaths annually from ARDS.11 Whereas in a third world setting, from 1046 patients admitted to a Rwandan hospital over 6 weeks, 4% (median age 37 years) met modified ARDS criteria. Only 30.9% of patients with ARDS were admitted to an ICU, and hospital mortality was 50.0%. This study used the Kigali modification of the Berlin definition: without a requirement for PEEP, hypoxia threshold of SpO2/FiO2 less than or equal to 315, and bilateral opacities on lung ultrasound or chest radiograph.12
The recently published LUNG SAFE trial was designed to study prospectively the performance of the Berlin definition and to reflect modern management of ARDS. To those ends, the investigators recorded admissions over 4 weeks to 459 ICUs in 50 countries over 5 continents including 29 144 patients. In total, 3022 (10.4%) cases fulfilled ARDS criteria, including almost a quarter of those supported with invasive mechanical ventilation.13 Despite this relatively high prevalence and the study’s focus on ARDS, the syndrome was recognised in only half of the mild ARDS group. Furthermore, in a study that reported on 815 patients with at least one risk factor for ARDS who were admitted to one of 3 Spanish hospitals over 4 months, 15 out of 53 patients (28%) were not admitted to an ICU suggesting that LUNG SAFE may have underestimated both ARDS incidence and overlooked diagnoses.14
Survivors commonly suffer from muscle weakness and neuropsychiatric problems, such that fewer than 50% have returned to work 12 months after leaving intensive care.15 However, it is unusual for ARDS survivors to be significantly limited by chronic respiratory failure. Therefore, ARDS is important both clinically and financially, because it is a not uncommon contributor to the deaths of critically ill patients of all ages and because survivors carry on suffering from the sequelae of critical illness long after they leave hospital.16
Pathophysiology
The pathophysiology of ARDS results from acute inflammation affecting the lung’s gas exchange surface, the alveolar-capillary membrane.1 Increased permeability of the membrane associated with the recruitment of neutrophils and other mediators of acute inflammation into the airspace manifests as high permeability pulmonary oedema. The resulting acute inflammatory exudate inactivates surfactant leading to collapse and consolidation of distal airspaces with progressive loss of the lung’s gas exchange surface area. This would be compensated for by hypoxic pulmonary vasoconstriction, if the inflammatory process did not also effectively paralyse the lung’s means of controlling vascular tone, thereby allowing deoxygenated blood to cross unventilated lung units on its way to the left heart. The combination of these two processes causes profound hypoxaemia and eventually type 2 respiratory failure as hyperventilation fails to keep pace with carbon dioxide production.
Diagnosis
Any diagnostic strategy for ARDS is sufficiently dependent on local factors, such as the prevalent causes of infectious pneumonia and access to imaging modalities, that a single protocol cannot be recommended. An exemplar from a tertiary referral centre used to dealing with complex and very severe cases is included (figure 1, p43–44). There are two main broad categories of condition that resemble ARDS but have a distinct pathophysiology: first, cardiovascular conditions of rapid onset including left heart failure, right-to-left vascular shunts usually with some lung pathology and major pulmonary embolism; second, lung conditions which develop more slowly than ARDS, for example, interstitial lung diseases (especially acute interstitial pneumonia), bronchoalveolar cell carcinoma, lymphangitis and the pulmonary vasculitides.
Sixty-day and hospital mortality comparing LTV and HTV mechanical ventilation in adult patients with ARDS. ARDS, acute respiratory distress syndrome; HTV, higher tidal volume; LTV, lower tidal volume.
Technical summary
The guidelines for the management of adult patients with ARDS were created by a multidisciplinary writing group constituted by the Joint Standards Committee of the Faculty of Intensive Care Medicine and the ICS. All group members, including lay members, are coauthors of the guideline. The group first met in 2013 and completed the guidelines in 2018. The guidelines have undergone both independent external peer review and input from stakeholder organisations.
The process for guideline creation adhered to that of the National Institute for Health and Care Excellence (NICE). In brief, the writing group first performed a scoping exercise on the topic, having decided that the focus should be on effective treatment interventions. Ten topics were chosen based on existing guideline recommendations and the experience of committee members. These included:
Corticosteroids.
ECMO.
ECCO2R.
Fluid strategy.
High-frequency oscillation ventilation (HFOV).
Inhaled vasodilators (iVasoD).
Lung protective ventilation: tidal volume (Vt).
Neuromuscular blocking agents (NMBA).
PEEP.
Prone positioning.
Each topic was developed into a full protocol using the PICO (Population, Intervention, Comparison, Outcome) formulation. Search strategies for each topic were then developed by the group information expert with a focus on systematic reviews (SR) and meta-analyses (MA). Each topic was assigned to two group members with one acting as topic lead. High-quality MA and SR were selected and the references placed in an Endnote database. Preselected outcome data were extracted from these reviews, using the most up-to-date MA where possible. Data from older MA were used if not all the preselected outcomes could be extracted from the most recent MA.
The guidelines used the internationally recognised GRADE methodology.17 Group members received training on the GRADE process and were given a resource pack, which included a practical guide which was created in- house. GRADE makes recommendations based on patient centred and predetermined outcomes. It does not judge the quality of individual randomised controlled trials (RCTs) but makes quality assessments on the predetermined outcomes, which, where possible, are extracted from published MA.18
The following outcomes were chosen by the writing group as either of critical or high importance using the GRADE methodology:
Mortality (28 day, hospital and 6 month): Critically important.
Mortality (1 year): Critically important.
Length of stay (ICU and hospital): Important.
Quality of life (at 3 months): Critically important.
Quality of life (at 6 months and 1 year): Important.
Harms (at 3 months, 6 months and 1 year): Important.
GRADE has a transparent methodology and guides recommendations based on the evidence collected. In reality, treatment recommendations are a graduation. However, in order to aid clinical decision-making, GRADE converts the continuum into five mutually exclusive categories.17 Recommendations are therefore categorised as strongly in favour, weakly in favour (or conditional), strongly against or weakly against (conditional). Finally, a research recommendation can be made where the estimate of the magnitude of effect and its boundaries were so imprecise and wide that further research is likely to make a fundamental change to a recommendation. Recommendations were made by the whole writing group. The lead author would present the data and suggest a recommendation, using GRADE methodology, based on the balance of benefits and harms as detailed in the GRADE tables and evidence. The group would then debate the topic and reach a consensus, based on the opinion of the majority.
Corticosteroids
PICO question
In adults with ARDS, does the use of corticosteroids, compared with standard care, affect survival and selected outcomes?
Study identification
The search strategy was predefined as per the online appendix C (https://www.ficm.ac.uk/sites/default/files/appendix_c_-_search_strategies.pdf). The role of corticosteroids in ARDS has been studied in RCT both in populations at risk of developing ARDS and in the established syndrome. These prevention and treatment trials have been separately analysed in most SR with MA; the results of the former have been excluded from this analysis. Eight, high quality SRs with MA, performed between 2008 and 2014, were identified (see PRISMA chart in online appendix A (https://www.ficm.ac.uk/sites/default/files/appendix_a_-_prisma_diagrams.pdf).19–26 A total of eight RCTs performed between 1985 and 2007 were included in these reviews. The largest single study enrolled only 180 patients.
A GRADE Summary of Findings table is shown below based on critical and important outcomes (table 1). A full GRADE evidence table can be found as part of the online appendix B (https://www.ficm.ac.uk/sites/default/files/appendix_b_-_grade_evidence_tables.pdf).
Corticosteroids compared to placebo for ARDS
Analysis of outcomes
Mortality
A MA of hospital mortality alone was presented in two SRs,19 21 while combined data on both hospital and 60-day mortality were presented in another SR.20 The quality of evidence supporting the relative risk (RR) of 0.51 (95% CI 0.24 to 1.09) in hospital mortality with steroids was very low21 (see GRADE evidence profile table 1). There was a serious risk of bias with only 75% of the Cochrane risk of bias recommendations followed. Inconsistency was also serious with point estimates varying widely, confidence intervals overlapping, a lack of consistent direction of effect and significant heterogeneity (I2 52%). Imprecision was also serious. A posthoc power calculation suggests that the pooled studies only had an approximately 65% power and a sample size calculation based on the reported effect size suggested that sample size was inadequate (predicted sample size of 474; actual pooled sample size of 341 for hospital mortality). This is likely to be an underestimate of the sample size required, as the effect size is likely to be smaller than the pooled data suggest due to heterogeneity of the studies.
A further issue is the fact that the majority of these studies were performed in the prelung protection strategy era. The largest ARDS Network steroid study, LASARUS, changed its ventilation protocol during the study to reflect the results of the ARDS Network ARMA low tidal volume study.27
The other hospital mortality analysis also reported low-quality data with an estimated RR of 0.62 (0.23–1.26).19 Combining hospital and 60-day mortality gave a RR estimate of 0.91 (0.71–1.18) with serious inconsistency and indirectness issues including the fact that this was a pooled estimate of both preventative and treatment studies.20
Length of stay
A MA of hospital length of stay was presented in one SR and MA.22 A mean reduction of 4.8 days with steroid treatment was reported but the overall quality of the studies was very low.
Quality of life
This was not reported in the Included MA.
Economic data
No economic data were published in the trials included.
Treatment harms
Potential harms of treatment with steroids included excess hospital acquired infections, ICU acquired weakness and delirium. The only available MA reported a composite analysis of infection, ICU acquired weakness, diabetes, gastrointestinal bleeding and other complications.21 The RR reported was 0.82 (0.5–1.36) but the quality of the trials was low.
GRADE recommendation statement
The use of corticosteroids in established ARDS should be the subject of a suitably powered, multicentre RCT with long-term follow-up (GRADE Recommendation: research recommendation).
GRADE recommendation justification
Current evidence includes the possibility of substantial patient benefit and the risk of harm appears small, although the group noted that the trials did not include longer term follow-up of survivors. However, the evidence is of low to very low quality from clinical trials, which were mostly conducted before the current era of lung protective ventilation. In addition, the lack of sufficient power in any individual study or in the combined MA and the heterogeneity of the dose, timing and agent used also influenced the decision. The group believed that a position of equipoise exists and the research recommendation reflects this view.
As a caveat, it is worth mentioning that specific steroid responsive disorders may mimic ARDS, for example, pneumocystis jiroveci pneumonia, acute eosinophilic pneumonia and diffuse alveolar haemorrhage.
Implications for future research
A large, multicentre study on steroids in established ARDS is currently planned.
Extracorporeal membrane oxygenation
PICO question
In adults with ARDS, does the use of ECMO, compared with standard care affect survival and selected outcomes?
Study identification
The search strategy was predefined as per online appendix C. Eight, relevant SR were identified, of which three included a MA28–30 (see PRISMA chart in online appendix A). When analysing results, we used the most recent SR with MA that considered the outcome in question.29 The selected SR with MA included only two RCTs of ECMO in adults with ARDS. These RCTs were published in 1979 and 2006 and included a total of 270 participants.31 32 The older RCTs32 did not combine the use of ECMO with protective low tidal volume mechanical ventilation and so is of little relevance to current practice. Data from this RCT and RCTs investigating the use of extracorporeal carbon dioxide removal (ECCO2R)33 34 were excluded. By contrast, we included in our de novo MA two quasi-RCT, which used genetic matching with replacement to identify control subjects and compared these with patients supported with ECMO in a total of 346 patients, all with pandemic H1N1 2009 influenza A.35 36
A GRADE Summary of Findings table is shown based on critical and important outcomes (table 2). A full GRADE evidence table can be found as part of the online appendix B.
ECMO compared to standard care for ARDS
Analysis of outcomes
Mortality
Hospital mortality was studied in two quasi-RCT in patients with H1N135 36 and hospital mortality was combined with mortality up to 6 months after hospital discharge in the RCT (CESAR) that recruited a general adult population with severe ARDS.31 Point estimates consistently showed a reduction in mortality in patients supported with ECMO: the risk ratio for hospital mortality was 0.64 (0.51–0.79). However, owing to the potential bias and lack of generalisability in the quasi-RCTs, the quality of evidence was deemed to be very low.
Length of stay
This was not reported in the included MA.
Quality of life
This was not reported in the included MA.
Economic data
This was not reported in the included MA. The CESAR study alone included both cost utility and cost effectiveness analyses enabling investigators to predict a lifetime cost per quality-adjusted life year (QALY) for ECMO of £19 252 (CI 7622 to 59 200) at a discount rate of 3.5%.31
Treatment harms
The use of ECMO is associated with the risk of serious bleeding, although this has not been universally reported or consistently defined in published studies. The risk ratio for bleeding associated with ECMO was 11.44 (3.11–42.06). The quality of evidence was deemed to be very low because data were available from two non-randomised studies that only included patients with ARDS associated with influenza A (H1N1).35 36
Grade recommendation statement
We do not recommend the routine use of ECMO for all patients with ARDS (GRADE Recommendation: weakly against). We suggest the use of ECMO with lung-protective mechanical ventilation in selected patients with severe ARDS (GRADE Recommendation: weakly in favour).
Grade recommendation justification
The use of ECMO in selected adults suffering severe ARDS (defined as a Lung Injury Score of 3 or more or pH<7.20 due to uncompensated hypercapnoea),was given a weakly positive recommendation based on very low quality evidence. The most widely used indications for ECMO are those reported in the CESAR study.31 There is a paucity of data to make this judgement: one RCT remains after excluding studies including patients supported with ECCO2R and one RCT from 1979 in which mechanical ventilation was not protective. Arguably the predominant mechanism through which ECMO may confer a benefit is by enabling the dramatic reduction of ventilation volumes and pressures, thereby mitigating ventilator-associated lung injury (VALI).
Scant evidence, again of very low quality, suggested an increased risk of bleeding associated with the use of ECMO: consistent with data from the extracorporeal life support organisation (ELSO), which publishes its registry data from around 300 centres world-wide. The incidence of serious bleeding (approximately 15% overall) and intracranial haemorrhage (3.9%) associated with the use of veno-venous ECMO for respiratory failure in adult patients based on data from the ELSO registry from its inception in 1989 to 2016 has recently been reported.37
Extracorporeal carbon dioxide removal
PICO question
In adults with ARDS, does the use of ECCO2R, compared with standard care affect survival and selected outcomes?
Study identification
The role of ECCO2R in ARDS has been studied in two RCTs in patients with ARDS enrolling 119 subjects. These trials have been analysed in SR without MA: there were significant difference between the studies in both ECCO2R technique and conventional ventilator strategy. Consequently, the SR was not able to perform a meaningful MA. There were two RCTs performed between 1994 and 2013.33 38
A GRADE Summary of Findings table is shown based on critical and important outcomes (table 3). A full GRADE evidence table can be found as part of the online appendix B.
ECCO2R compared to standard care for acute respiratory distress syndrome
Analysis of outcomes
Mortality
The risk of bias in the two RCTs was low. Both studies were stopped early following planned interim analyses and concluded that any difference between control and intervention groups was too small to be demonstrated. One trial enrolled 79 out of a planned 12033 and the other 40 out of a planned 60.38 In one RCT, in-hospital mortality was 17.5% and 15.4% in the intervention and control groups, respectively.33 The other RCT reported 30-day mortality in the intervention group of 66.6% and 57.9% in the control.38 These were not significantly different.
Length of stay
This was not reported in the included SR.
Quality of life
No trial reported on quality of life.
Economic data
No trial reported on economic data.
Treatment harms
Potential harms of treatment with ECCO2R included bleeding and thrombosis. Complications were dependent on the type of ECCO2R used with approaches which required arterial cannulation reporting an incidence of arterial injury from 0% to 25%.39 Blood transfusion requirements were also increased in the ECCO2R group.39
Grade recommendation statement
The use of ECCO2R in established ARDS should be the subject of a suitably powered multicentre RCT with long term follow-up and economic analysis (GRADE Recommendation: research recommendation).
Grade recommendation justification
Current evidence is extremely limited and mainly consists of non-randomised prospective and retrospective trials.40–44 The substantial differences between the techniques for both ECCO2R and conventional ventilation make the two RCTs incomparable. However, there is evidence that ECCO2R can allow ventilation with tidal volumes lower than currently recommended for ARDS and the potential benefits of this approach should be tested in an appropriately designed RCT. The group believed that a position of equipoise exists and the research recommendation reflects this view.
Implications for future research
A large, multicentre study evaluating veno-venous ECCO2R to facilitate lower tidal volume ventilation in patients with acute hypoxaemic respiratory failure is currently ongoing, the REST (protective ventilation with veno-venous lung assist in respiratory failure) trial (http://www.nictu.hscni.net/rest-trial). NICE guidelines on the use of ECCO2R encourage clinicians to recruit patients to the REST trial.
Fluid management
PICO question
In adults with ARDS, does the use of a conservative fluid strategy, compared with a liberal fluid strategy or standard care, affect survival or selected outcomes?
Study identification
The search strategy was predefined as per the online appendix C. Of four SR identified,45–48 one recent high quality SR with MA addressing the question of optimal fluid strategy in ARDS was included.45 This review included patients with ARDS, sepsis and SIRS, although subgroup data were available for ARDS. The review included data from five RCTs in ARDS49–53 performed between 2002 and 2014, and ranging from 29 to 1000 participants. Significant clinical heterogeneity was evident between these studies in terms of intervention strategies, fluid balance achieved and outcome reporting. Conservative fluid strategies included protocolised diuretic use, with49 50 or without51 hyperoncotic albumin solutions, minimisation of fluid intake51 and the use of extravascular lung water (EVLW) measurements to guide fluid therapy.52 Liberal fluid strategies varied from a protocolised fluid administration strategy, which approximated the usual care arm of previous large trials in ARDS,51 use of furosemide without hyperoncotic albumin50 and use of pulmonary capillary wedge pressure (PCWP) to guide fluid administration.52 One study did not define conservative and liberal fluid strategies in detail.53
A GRADE summary of findings table is shown for critical and important outcomes (table 4). A full GRADE evidence table can be found as part of the online appendix B.
Conservative compared to liberal fluid management for ARDS
Analysis of outcomes
Mortality
Heterogeneity in outcome reporting was evident, with two studies reporting mortality at 30 days49 50 and three at 60 days;51–53 the pooled results showed no effect of fluid balance strategy on mortality.
Moderate quality evidence supported an RR of 0.91 (95% CI 0.77 to 1.08) for mortality using a conservative rather than a liberal fluid strategy. Although two of the RCTs included were at high or uncertain risk of bias,52 53 these studies included only 129 of 1206 patients, and thus overall no serious risk of bias was deemed to be present. Serious indirectness was present, in that various treatment regimens were compared, including a comparison of hyperoncotic albumin versus placebo as an adjunct to diuretic therapy,50 and of EVLW-guided with PCWP-guided fluid therapy.53 Exclusion of these studies made little difference to the point estimate. As confidence intervals around the point estimate were wide, neither clinically important benefit nor harm could be excluded.
Length of ICU stay
Very low quality evidence for a reduction in length of ICU stay with a conservative fluid strategy was provided by two small RCTs including 129 patients.52 53 Both studies were at very serious risk of bias due to lack of blinding and other methodological flaws. One study52 compared EVLW-guided with PCWP-guided fluid therapy, neither of which is commonly used clinically, and a clinically important difference in fluid balance between groups was absent. The population, intervention and comparator in the other study were not reported in detail.53 The small number of patients in these studies also led to very serious imprecision.
Length of hospital stay
A single RCT50 provided low-quality evidence for the absence of an effect of fluid strategy on length of hospital stay. Very serious imprecision was present due to a lack of statistical power to exclude a clinically important difference on this outcome.
Quality of life
No trial reported on quality of life.
Economic data
No trial reported on economic data.
Treatment harms
Acute kidney injury incidence
Incidence of acute kidney injury (AKI) was felt to be important as this represents a potential harm associated with a conservative fluid strategy. A single large RCT51 provided low quality evidence for similar numbers of AKI-free days with conservative and liberal fluid strategies, and a posthoc analysis of this trial54 suggested a reduction in AKI incidence with a conservative fluid strategy using creatinine measurements corrected for changes in volume of distribution.
Requirement for renal replacement therapy
It was considered that requirement for renal replacement therapy (RRT) represented a potential harm from a conservative fluid strategy. Moderate quality evidence for a reduction in the requirement for RRT with a conservative fluid strategy was provided by a single large RCT51 (RR 0.71, 95% CI 0.50 to 0.99).
Cognitive dysfunction
A posthoc analysis of a small subgroup of patients from the FACTT trial found conservative fluid strategy to be an independent risk factor for long-term cognitive dysfunction following ARDS.55 One RCT of uncertain risk of bias found better cognitive outcome scores with conservative fluid strategy than with liberal fluid strategy,53 although the duration of follow-up and details of the intervention were not described.
Grade recommendation statement
We suggest the use of a conservative fluid strategy in patients with ARDS (GRADE recommendation: weakly in favour).
Grade recommendation justification
Despite the low quality of evidence for the majority of outcomes, and the results being driven largely by a single trial,51 conservative fluid management may be beneficial without evidence of harm. We therefore suggest that in adult patients with ARDS, clinicians consider the use of a conservative fluid strategy which uses fluid restriction, diuretics and possibly hyperoncotic albumin to avoid a positive fluid balance in preference to a liberal fluid strategy.
Ventilation high frequency oscillatory
PICO question
In adults with ARDS, does the use of HFOV, compared with standard care, affect survival and other selected outcomes?
Study identification
The search strategy was predefined as per online appendix C. The role of HFOV in ARDS with moderate to severe hypoxaemia has been studied in six RCTs published between 2002 and 2013.56–61 Two recent RCTs enrolled a disproportionate number of patients—1343 out of 1608 patients (795 patients in one and 548 in the other). There have been an additional two RCTs of HFOV combined with tracheal gas insufflation.62 63 These trials have been analysed in three SRs with MA.64–66
Data were analysed from two of these: the most recent MA was used first,64 supplemented with additional data from previous studies.65 One MA was excluded as it combined results of RCTs with HFOV and tracheal gas insufflation with those of RCTs with HFOV alone.66
A GRADE Summary of Findings table is shown below based on critical and important outcomes (table 5). A full GRADE evidence table can be found as part of online appendix B.
HFOV compared to usual care for ARDS
Analysis of outcomes
Mortality
The RR of death associated with HFOV was 1.218 (0.925 to 1.604). The evidence was judged to be of moderate quality.64 Of the RCTs contributing to the two MAs, five demonstrated no difference in mortality between HFOV and conventional ventilation,56–61 while one of the larger RCTs demonstrated increased mortality in the HFOV arm.59 The overall risk of bias in included studies was low with the exception of two studies where crossovers accounted for more than 10% of the study group.56 58 Inconsistency was serious with point estimates varying widely, confidence intervals overlapping, a lack of consistent direction of effect and significant heterogeneity (I2=63.1%, p=0.028).
Length of stay
This was not reported in the included SR.
Quality of life
No trial reported on quality of life.
Economic data
No trial reported on economic data.
Treatment harms
Potential harms of HFOV were reported including barotrauma, hypotension and oxygenation failure. The RR of barotrauma was reported from four studies enrolling 752 subjects as 1.205 (95% CI 0.834 to 1.742); however, the studies used a variable definition of barotrauma.64 The RR of hypotension was reported as 1.326 (95% CI 0.271 to 6.476) and these data were derived from three studies enrolling 237 patients.64 Oxygenation failure in the MA included 757 patients from three studies with a RR for HFOV of 0.557 (95% CI 0.351 to 0.884).64
Grade recommendation statement
We do not recommend the use of HFOV in the management of patients with ARDS (GRADE recommendation: strongly against).
Grade recommendation justification
The use of HFOV for the management of ARDS was given a GRADE recommendation of strongly against based on moderate quality evidence. Current evidence from multiple RCTs demonstrated no benefit from HFOV and one RCT demonstrated an increase in mortality with HFOV.
Inhaled vasodilators
PICO question
In adults with ARDS, does the use of inhaled vasodilators (iVasoD), compared with standard care, affect survival and selected outcomes?
Study identification
The search strategy was predefined as per online appendix C. The role of the iVasoD nitric oxide (iNO) in the management of ARDS has been assessed in multiple RCT, which have been analysed in subsequent SR with MA. No studies examining the role of nebulised prostacyclin in adults with ARDS were identified by Cochrane reviewers in 2010.67
Three SR with MA were identified from which data were analysed (see PRISMA chart in online appendix A).68–70 Mortality data were analysed from nine RCTs71–79 published between 1998 and 2004, including 1142 participants. Exclusion criteria for RCT included: >50% cross-over between iNO and placebo groups and unequal distribution of other rescue therapies between treatment and control groups. Limited information on possible harms was available: data from four RCTs71 73 77 78 provided specific information regarding nephrotoxicity associated with the use of iNO.
A GRADE Summary of Findings table is shown below based on critical and important outcomes (table 6). A full GRADE evidence table can be found as part of online appendix B.
iVasoD compared to placebo or usual care for ARDS
Analysis of outcomes
Mortality
Mortality at hospital discharge was used for analysis where available. Otherwise these data were combined with mortality at discharge from the ICU or 28–30 days after randomisation. The quality of evidence supporting the RR of 1.10 (95% CI 0.94 to 1.29: p=0.24) in the first treatment analysis was low (see GRADE evidence profile table). In only 3/9 studies72 78 79 was placebo gas (nitrogen) administered to the control group, creating a serious risk of bias in the other six studies. There was serious indirectness in the nine studies analysed owing to variability in inclusion criteria including marked deviation from AECC criteria for diagnosing ALI/ARDS and variable iNO treatment regimens. Data from studies using different doses of iNO were combined. These variable doses and duration of treatment, which may be considered to be too high and too long respectively, constitute a serious source of indirectness. Consistency was good with confidence intervals overlapping, a consistent direction of effect and a very low heterogeneity.70
Subgroup analysis from 7/9 trials did not support the hypothesis that iNO conferred a survival benefit in patients with severe ARDS (PaO2 to FiO2 ratio of <20 kPa).
Length of stay
No MA available.
Quality of life
No trial reported on quality of life.
Economic data
No trial reported on economic data.
Treatment harms
The administration of iNO was associated with an increased incidence of renal dysfunction in four trials representing 80% of the patients recruited into the nine studies analysed above (risk ratio 1.50, 1.11 to 2.02). The quality of the evidence supporting the association was judged to be low based on the factors outlined above and the variable criteria used to define renal dysfunction, although the consistency between trials was good.
Grade recommendation statement
We do not suggest using iNO in patients with ARDS (GRADE Recommendation: weakly against).
Grade recommendation justification
The recommendation that iNO is not used for adult patients with ARDS is based on low quality but consistent evidence suggesting a lack of mortality benefit and an association with renal dysfunction. While the studies examining the role of iNO in ARDS are imperfect, further trials would be given a low priority. The possible use of iNO in patients with severe right ventricular dysfunction or extreme hypoxaemia for short periods, while more long-term rescue strategies (such as ECMO) are instituted, fall outside the scope of this guideline.
Mechanical ventilation at lower tidal volume
PICO question
In mechanically ventilated adult patients with ARDS, do lower tidal volumes compared with higher, conventional tidal volumes affect survival and other related outcomes?
Study identification
The search strategy was predefined as per online appendix C. Seven, full text, SR were assessed for eligibility. We excluded four reviews: three did not contain the full complement of published trials80–82 and one contained studies of patients without ARDS.83 The remaining three reviews84–86 each contained the six RCT that met the PICO inclusion criteria. We extracted the mortality data provided by Petrucci 201385 (the most recent published review) to the GRADE profiler. In addition, we reviewed the published papers and extracted additional outcomes that were relevant to the guidelines, but not reported in the three SR.
The Petrucci 2013 review included six multi-centre RCT published from 1998 to 2006 that included a total of 1297 patients. Within-trial sample sizes ranged from 52 to 861 patients. Trials were conducted in North and South America and Europe. Four trials87–90 compared lower tidal volumes (range <6–8 mL/kg) and restricted airway pressures (plateau pressure<30 cmH2O) with higher tidal volumes (range 9–15 mL/kg) and airway pressures (plateau pressure <50–60 cmH2O). The Amato 1998 92 and Villar 2006 93 trials compared lower tidal volume with higher PEEP, where possible set just above the lower inflection point of a pressure-volume curve, and higher tidal volume with lower PEEP: these studies investigating the composite intervention of lower tidal volume and higher PEEP were analysed separately.
We provide Forest plots to show the separate MA for the comparisons of (1) lower versus higher tidal volumes with similar PEEP and (b) lower tidal volumes with higher PEEP versus higher tidal volume with lower PEEP. A GRADE Summary of Findings table is shown based on critical and important outcomes (table 7). A full GRADE evidence table can be found as part of online appendix B.
Lower tidal volume compared with higher tidal volume (at similar PEEP) for ARDS
Analysis of outcomes
Lower versus higher tidal volume with similar PEEP
Mortality
In this comparison, four studies reported mortality at varying time-points: 60 days91 and hospital discharge (figure 1).87–89 Pooled data from the four trials showed no significant difference between lower and higher tidal volume groups in risk of death including all time-points (RR 0.87, 95% CI 0.75 to 1.01, p=0.07) with moderate, but non-significant heterogeneity (I2 48%, p=0.13). Pooled data for hospital mortality showed a statistically significant reduction in risk of death (RR 0.83, 95% CI 0.71 to 0.98, p=0.02) associated with lower tidal volume ventilation, whereas a non-significant increase in risk was found at 60 days (RR 1.23, 95% CI −0.80 to 1.89, p=0.35) based on data from a single study with relatively few patients, in which body weight was not corrected according to the ideal or predicted standard (http://www.ardsnet.org/files/pbwtables_2005-02-02.pdf).
ICU length of stay
The pooled effect from two studies90 91 showed no significant difference in length of ICU stay (mean difference 4.79 days, 95% CI −2.06 to 11.63, p=0.17) (figure 2).
ICU length of stay comparing LTV and HTV mechanical ventilation in adult patients with ARDS. ARDS, acute respiratory distress syndrome; HTV, higher tidal volume; ICU, intensive care unit; LTV, lower tidal volume.
Hospital length of stay
There was no difference in hospital length of stay reported by one study90 (mean difference 6.30 days, 95% CI −7.53 to 20.13, p=0.37).
Quality of life
No trial reported on quality of life.
Economic data
No trial reported on economic data.
Lower tidal volume with higher PEEP versus higher, conventional tidal volume with lower PEEP
Mortality: 28-day, ICU and hospital
Two studies reported mortality. At 28 days, one study92 showed a significant reduction in risk of death in the lower tidal volume and higher PEEP group (RR 0.54, 95% CI 0.31 to 0.91, p=0.02). Similarly, pooled data from two studies showed a significant reduction in risk of ICU mortality (RR 0.57, 95% CI 0.40 to 0.82, p=0.002) and hospital mortality (RR 0.62, 95% CI 0.44 to 0.87, p=0.006).92 93 In both cases, the evidence was downgraded to low because of imprecision (relatively few patients, 156 in total), and indirectness of evidence (methodological flaws—body weight was not corrected in one study; and lack of generalisability based on the unusually high mortality rate of the conventional ventilation group).
Grade recommendation statement
We recommend the routine use of lower tidal volumes for the management of patients with ARDS (GRADE Recommendation: strongly in favour).
Grade recommendation justification
The recommendation to use lower tidal volume (less than or equal to 6 mL/kg predicted body weight) ventilation with a plateau pressure less than or equal to 30 cmH2O is strong despite moderate quality of evidence for hospital mortality and barotrauma, but low quality of evidence for 60-day mortality. The evidence was downgraded for serious indirectness for hospital mortality, and for inconsistency and imprecision for 60 day mortality. For example, the beneficial effects of low tidal volume ventilation were only seen in one large trial and the means of managing respiratory acidosis in the ARDS Network ARMA trial87 is not generally applied. However, a lack of adverse effects associated with the intervention, strong mechanistic rationale for its use94 and supportive data from ARDS prevention studies95 have resulted in its universal acceptance as a gold standard of care.
Neuromuscular blocking agents
PICO question
In adults with ARDS, does the use of NMBA, compared with standard care, affect survival and selected outcomes?
Study identification
The search strategy was predefined as per online appendix C. The only NMBA studied in an RCT considering outcomes relevant to our PICO question was cisatracurium besylate. Four SR were identified,96–99 published between 2012 and 2015, of which only two included MA.96 97 When analysing results, we used the most recent SR with MA96 that considered the outcome in question. The two selected SR with MA included the three RCTs of NMBAs that were identified, both of which compared a continuous 48 hours infusion of cisatracurium with standard care. These RCTs were published between 2004 and 2010 and included a total of 431 participants from 20 French ICUs.
A GRADE Summary of Findings table is shown based on critical and important outcomes (table 8). A full GRADE evidence table can be found as part of online appendix B.
NMBAs compared to placebo for ARDS
Analysis of outcomes
Mortality
Mortality (pooled 28 day, ICU and hospital mortality) was reported in all three RCTs100–102 with point estimates showing a reduction in mortality at each of these time points. However, in each of these RCT, the 95% CI for the risk ratio reached or crossed the no effect line. When mortality data from these RCTs were pooled in MA (with a total of 431 participants), the CI was narrowed to show a significant reduction in mortality at each of these time points. The risk ratios for 28 day, ICU and hospital mortality were 0.66, 0.70 and 0.72, respectively, suggesting a significant reduction in the risk of mortality with this intervention.
Although these results showed a good level of consistency and precision, there are important concerns over the risk of bias and indirectness in the contributing RCT. All three studies, which were conducted by the same team of investigators in France, have been criticised for the lack of effective blinding of caregivers to study group allocation. In two of the studies,100 102 no attempt was made to blind caregivers while, in the third,101 it is questionable whether blinding was effective. It has also been noted that there is considerable overlap of authorship of the most recent SR and the contributing RCT. One of the contributing RCTs101 included only patients with severe ARDS (P/F ratio<20 kPa) within the first 48 hours, leading to our assessment of ‘serious’ indirectness of the findings for ARDS as a whole.
Length of stay
This was not reported in the included SR.
Quality of life
This was not reported in the included SR.
Economic data
This was not reported in the included SR.
Treatment harms
A key concern for the use of NMBA in ICU is the presumed risk of increased ICU-acquired weakness with their use. Although the risk of ICU-acquired weakness was not found to be significantly increased on MA (RR 1.08; 95% CI 0.83 to 1.41), these findings are severely limited by the lack of robust screening measures in two of the contributing RCT,100 102 and by the lack of follow-up beyond ICU discharge in the final RCT101
Grade recommendation statement
We do not suggest using NMBAs for all patients with ARDS (GRADE Recommendation: weakly against). We suggest the use of cisatracurium besylate by continuous 48 hours infusion in patients suffering early moderate/severe ARDS (P/F<20 kPa: GRADE Recommendation: weakly in favour).
Grade recommendation justification
The use of cisatracurium besylate in adults suffering early, severe ARDS was given a weakly positive recommendation based on moderate evidence quality. The group felt it was appropriate to recommend this management protocol because it was the only one studied by RCT. Due to the nature of this intervention, it should only be given to patients who are adequately sedated and receiving invasive ventilation. As such, it would have been difficult to recruit patients with mild ARDS.
Although it is reassuring that in all three RCTs the point estimate of treatment effect indicated a survival benefit, it was only by pooling these data in MA that these findings reached statistical significance. There are also concerns over the ineffective blinding of caregivers to study group allocation in the clinical trials and concerns that the potential association of NMBA and ICU-acquired weakness was not studied in a robust manner.
Positive end-expiratory pressure
PICO question
In adult patients with ARDS, does mechanical ventilation with higher PEEP, compared with standard (lower) PEEP improve survival, and selected outcomes?
Study identification
The search strategy was predefined as per online appendix C. Six high-quality SR with MA were identified (see PRISMA chart in online appendix A). These used data from a total of seven clinical trials,92 93 103–107 published between 1998 and 2009. The largest single study enrolled 983 patients.105 Data from three MA form the basis of the recommendation,108–110 with the most recent used where possible for outcomes of interest. Where this MA did not provide information on relevant outcomes, alternative MA were used.
A GRADE summary of findings table is shown based on critical and important outcomes (table 9). A full GRADE evidence table can be found as part of online appendix B.
Higher PEEP compared to lower PEEP for ARDS
Analysis of outcomes
Mortality
A MA of hospital mortality alone was presented in the most recent SR assessing the impact of higher PEEP in ARDS.110 The quality of evidence supporting the RR of 0.90 (0.81–1.01) was deemed moderate, as there were different strategies used between the trials to set PEEP. The mean PEEP levels in each arm of the three studies are presented in table 10.
PEEP values at day 1 in clinical trials
Mortality within 28 days of randomisation was presented in the same MA, and the quality of evidence supporting the RR of 0.83 (0.67–1.01) was low, as the analysis included trials which incorporated low tidal volume ventilation in the high PEEP arm, while the control group were ventilated with a low PEEP, high tidal volume strategy.
Individual patient data MA of three RCT (table 10) evaluating high vs low PEEP showed a reduction in ICU mortality (up to day 60) in patients with moderate or severe ARDS (P/F<27 kPa): RR 0.85 (0.76 to 0.95). There is moderate quality of evidence supporting this assessment, again because different strategies to set PEEP levels were used. This analysis is supported by a MA of three randomised trials that, with low quality of evidence, reported a reduced ICU mortality in patients with moderate or severe ARDS (RR 0.67 (0.48 to 0.95).110
Additional individual patient data MA evaluating the effect of high PEEP on hospital mortality in three studies reported a RR 0.90 (0.81 to 1), with evidence supporting this finding regarded as moderate (see GRADE evidence profile table).108
Length of stay
In a MA of two trials, a high PEEP strategy was not associated with a significant reduction in ICU-free days (0.04 (95% CI −1.03 to 1.1)). This is supported by a moderate evidence base given the wide confidence interval that extends beyond the 25% threshold.
Quality of life
This was not reported in the included SRs.
Economic data
This was not reported in the included SRs.
Treatment harms
A higher PEEP ventilation strategy was not associated with increased rates of air leaks (RR 0.97 (0.66 to 1.42)), with the evidence supporting this finding deemed very low because of the difference in tidal volume strategies assessed between the intervention and control arms of some studies, and the imprecision of the results.110
Grade recommendations
We suggest the use of high PEEP strategies for patients with moderate or severe ARDS (P/F ratio <27 kPa: GRADE Recommendation: weakly in favour).
Grade justification
We identified low-quality evidence to support the use of higher PEEP strategies in the ventilation of patients with moderate or severe ARDS. Evidence was downgraded because of inconsistency caused by differences between individual studies in the strategy to set the level of PEEP, while some trials compared lower tidal volume ventilation as part of a ventilator strategy that incorporated higher PEEP levels. The recommendation to consider the use of higher PEEP in patients with at least moderate ARDS is based on subgroup and individual patient data MA, providing less robust evidence than a RCT investigating higher PEEP in this patient group. The risk of barotrauma as a result of the use of higher PEEP for patients with at least moderate or severe ARDS cannot be excluded because this risk has not been quantified in this population. The quality of this evidence is also limited by inconsistency as the MA included trials of high PEEP with different tidal volume strategies.
Prone positioning
PICO question
In adults with ARDS, does the use of prone positioning, compared with standard care, affect survival and selected outcomes?
Study identification
The search strategy was predefined as per online appendix C. Fourteen eligible SRs investigating the effect of prone positioning in ARDS98 111–123 (see PRISMA chart in online appendix A) were identified. Twelve reviews included a MA.98 111–113 115 117–123 The most recently published of these was used for data extraction.120
A GRADE Summary of Findings table is shown based on available evidence for critical and important outcomes (table 11). A full GRADE evidence table can be found as part of online appendix B.
Prone positioning compared with standard care for ARDS
Analysis of outcomes
Mortality
Mortality (defined as overall mortality at the longest available follow-up) was significantly reduced with prone positioning (RR 0.9; 95% CI 0.82 to 0.96, 8 studies, 2141 patients) with very low quality of evidence supporting this RR. All trials demonstrated performance bias, because of the impossibility of blinding patients and carers with respect to the intervention. All trials also demonstrated detection bias, where outcome assessors were not blinded to intervention allocation. One RCT additionally demonstrated selection bias124 and three separate trials suffered from attrition bias125–127 according to the Cochrane risk of bias recommendations.128 Inconsistency was very serious, with varied point estimates, overlapping confidence intervals with high and significant levels of heterogeneity. There was also serious indirectness as the cohort of trials included subgroups receiving additional interventions known to demonstrate a mortality benefit.
Subgroup analysis demonstrated that prone positioning in combination with lung-protective ventilation (low tidal volume ventilation, 6–8 mL/kg body weight) demonstrated a significant reduction in mortality (RR 0.73; 95% CI 0.62 to 0.86) compared with patients receiving prone positioning and no lung-protective ventilation (RR 1.01; 95% CI 0.9 to 1.13), supported by moderate quality evidence. These findings may be influenced by inclusion of one trial enrolling a sizeable patient cohort with more severe ARDS (P/F ratio <20 kPa, FiO2>0.6)129 which showed larger differences in mortality rates between patients managed prone and supine in the setting of lung-protective ventilation.
Subgroup analysis based on the duration of prone positioning found that over 12 hours of prone positioning was associated with significantly reduced mortality (>12 hour, RR 0.75, 95% CI 0.65 to 0.87;<12 hour, RR 1.03, 95% CI 0.91 to 1.17), again supported by moderate quality evidence.
Length of stay
ICU length of stay was only examined in two older MAs.112 123 However, these data could not be extracted, as pooled analyses included either confirmed or potential paediatric data. No other trial examined hospital length of stay.
Quality of life
No trial reported on health-related quality of life.
Economic data
No trial reported on economic data.
Treatment harms
Overall, the pooled risk of any adverse event with prone positioning was significantly increased (RR 1.10; 95% CI 1.01 to 1.12). Where a more detailed analysis of adverse events was conducted, endotracheal tube displacement (RR 1.33; 95% CI 1.02 to 1.74), the incidence of pressure sores (1.23; 95% CI 1.07 to 1.41) and loss of venous access (RR 1.98; 95% CI 1.11 to 3.55) were significantly increased. However, this evidence was down-graded based on the risk of bias and imprecision in the trials evaluated.
Grade recommendation statement
We do not recommend the use of prone positioning for all patients with ARDS. We recommend the use of prone positioning for at least 12 hours per day in patients with moderate/severe ARDS (P/F ratio<20 kPa: GRADE recommendation: strongly in favour).
Grade recommendation justification
Current evidence includes the possibility of substantial patient benefit in terms of reduced mortality when combined with lung-protective ventilation and when delivered for at least 12 hours to patients with moderate/severe ARDS. Evidence for these findings was of moderate quality. The GDG noted the relative improvements in study design over the time course of publication of all eight trials, such that the most recently published focused enrolment on the most severe strata of patients with ARDS, and involved a multimodal intervention comprising lung-protective ventilation with prolonged prone positioning producing highly favourable outcomes.129 This observation provides the rationale for the strong classification of recommendation.
The possibility for substantial patient benefit must be considered in the context of a significant risk of occurrence of adverse events including endotracheal tube displacement, pressure sores and loss of venous access, although the evidence to support these findings was either low or very low. However, the GDG felt that these adverse events could be mitigated by ensuring that sufficient skilled personnel were in place to deliver and monitor the intervention.
Conclusion
Summary
Table 12 outlines the GDG’s synthesis of data for the Management of ARDS from relevant clinical trials.
Summary of the FICM/ICS Guidelines for the management of ARDS in adult patients
Discussion
The summary of the group’s recommendations emphasises the importance of avoiding VALI in patients with ARDS, as all of the interventions with positive recommendations apart from maintaining a conservative fluid balance, arguably act through this process. Despite at best moderate quality evidence by MA, we have strongly supported the use of low tidal volume and low airway pressure mechanical ventilation. This ventilation strategy is supported by results of the ARDS Network ARMA study,87 data from studies whose primary outcome was the prevention of ARDS and a large volume of evidence from preclinical and mechanistic studies. It is now so universally accepted that it is mandated for all patients in clinical trials of ARDS. When applied to patients with moderate/severe ARDS for at least 12 hours per day, prone positioning was also strongly recommended because the most recent studies focused enrolment on the most severe strata of patients with ARDS and involved a multimodal intervention comprising lung-protective ventilation with prolonged prone positioning producing highly favourable outcomes.129 By contrast, despite a strong theoretical rationale as a means of preventing VALI, high frequency oscillatory ventilation was ineffective or deleterious in two large studies leading to our recommendation strongly against its use.
While broadly similar recommendations for the management of ARDS have been produced, many questions remain. Fundamentally the parameters characterising optimal protective mechanical ventilation are unknown, as are the optimal means of achieving them. We have recommended targeting <6 mL/kg ideal body weight (IBW), but, based on the absence of evidence of a safe tidal volume threshold on retrospective reanalysis of the ARMA study130 and a dose-response effect seen in observational studies,131 it would be reasonable to recommend minimising tidal volume as far as possible. Similarly, analysing individual patient data from RCT concluded that driving pressure (plateau pressure minus PEEP) was a better predictor of outcome than tidal volume or plateau pressure alone.132 Finally, there is no consensus regarding the means used to optimise PEEP (oxygenation or various lung mechanical parameters) or to manage the respiratory acidosis that commonly accompanies protective ventilation.
In certain details, recent guidelines have diverged. We felt that the evidence supporting the role of recruitment manoeuvres was so poor and the concept so ill-defined that we were unable to make a recommendation. By contrast, the American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine group has given a conditional recommendation, although with low-to-moderate confidence.5 Similarly, our group did not consider airway pressure release ventilation owing to the paucity of high quality, relevant evidence, despite the knowledge that this ventilatory mode is widely used. Hopefully there will be sufficient evidence to justify including these interventions in the next version of the guidelines.
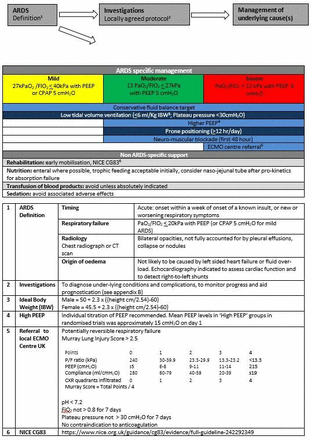
We have synthesised available evidence with the clinical practice of the GDG into a management algorithm (figure 1). Hence, for a patient presenting with for example severe ARDS, low tidal volume (<6 ml/kg IBW) and low plateau pressure (<30 cmH2O), mechanical ventilation using higher PEEP is recommended with the addition of neuromuscular blockade for the first 48 hours and prone positioning for at least 12 hours per day. After initial resuscitation of the circulation, a neutral or, if tolerated, a negative fluid balance target should be set. Consideration of escalation to extracorporeal lung support (ECMO or ECCO2R) is indicated by the failure to achieve adequate gas exchange using protective ventilatory settings as described above. To what extent is this synthesis evidence-based? While the individual components are to an extent evidence-based, the combination of interventions has evolved rather than being formally tested. For example, attempts have been made to test a so-called ‘open lung approach', by combining higher PEEP levels with low tidal volume ventilation both in early studies concentrating on low tidal volume ventilation, in subsequent PEEP trials and more recently in studies combining the use of recruitment manoeuvres and high levels of PEEP. The rationale for the open lung approach is that increasing airway pressure will increase the volume of ventilatable lung, thereby decreasing VALI and a large clinical trial was supported by encouraging pilot data. This Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) carried out in 1010 patients with severe ARDS surprisingly showed significantly higher 6-month mortality (65.3% vs 59.9%) in the intervention group.133 These data demonstrate the enduring value of large well-conducted clinical trials of complex interventions in this challenging patient group.
Unmet needs, research and future directions
We have made research recommendations for two interventions for adult patients with ARDS: corticosteroids and ECCO2R. Two international studies are currently examining the effects of ECCO2R combined with ultralow tidal volume ventilation (pRotective vEntilation with Veno-venouS Lung Assi (pRotective vEntilation With Veno-venouS Lung assisT in Respiratory Failure (REST, ClinicalTrials.gov NCT02654327) and SUPERNOVA: A Strategy of UltraProtective lung ventilation with Extracorporeal CO2 Removal for New-Onset moderate to seVere ARDS, whose pilot study has just been reported.
There are no disease modifying, drug therapies for ARDS. Drug development in this area is notoriously difficult, partly because ARDS is not a disease but a syndrome describing acute respiratory failure occurring de novo as a result of a wide variety of conditions. One strategy designed to increase the likelihood of positive clinical trials in ARDS is to select a less heterogeneous patient population—a step on the road to a personalised approach made at the expense of having a smaller pool of patients from which to recruit. Such splitting can be envisaged on the basis of readily identifiable predisposing causes (eg, influenza pneumonia, transfusion-related acute lung injury (TRALI) or systemic sepsis) or inherent patient characteristics, such as alcoholism or the expression of particular single nucleotide polymorphisms known to be associated with a predisposition to ARDS. The ultimate aim is to identify subgroups, so-called endotypes of ARDS that will predict a positive response to a certain class of therapy.134
Current management of ARDS is hampered by failure to diagnose the condition and to prevent iatrogenic harms. We need to heighten awareness of the diagnosis, particularly outside ICU, so that the opportunity to prevent progression of the syndrome is not missed. Research into prevention and treatment needs to be translated more effectively into the clinic. Biomarkers that confirmed the diagnosis highlighted patients with a poor prognosis and predicted that a positive response to a particular therapy would be invaluable in research and clinical care. For example, a validated bedside biomarker of VALI would facilitate the fine tuning of mechanical ventilation and could guide related decisions during the recovery phase of ARDS, for example, assessing the risk-benefit relationship between allowing spontaneous ventilatory modes with associated larger tidal volumes.
In order to discover effective drug therapies, continued investment in human studies that aim to elucidate the pathogenesis of ARDS is essential to identify clinically useful biomarkers and surrogate outcome measures.135 136 These investigations need to be performed with a view to designing a stepwise approach to testing novel therapeutics in this particularly challenging patient group.137
Finally, standardisation of outcome measures will help in the conduct and comparison of clinical trials and such work is underway, for example, the Core Outcomes for Ventilation Trials: the COVenT Delphi study (COMET registration: http://www.comet-initiative.org/studies/details/292). As reflected in the outcome prioritisation exercise carried out by the GDG, there is an increasing emphasis on the health of survivors of critical illness, which mandates that clinical trials include long-term outcomes and economic analysis that will inform the societal impact of intensive care medicine.
Management of ARDS in practice
Management
The essence of management of ARDS consists of optimising the diagnosis and treatment of underlying conditions, and the deployment of supportive measures that minimise iatrogenic injury and the consequences of severe critical illness (ie, secondary and tertiary prevention). We have combined these strategies with the outcome of the analysis of evidence relating to the topics selected in figure 3.
Analysis of evidence relating to the topics selected in this figure. ARDS, acute respiratory distress syndrome; CPAP, continuous positive airway pressure; ECMO, extracorporeal membrane oxygenation; PEEP, positive end-expiratory pressure.
Primary prevention
A common theme of research into critical illness has been the increasing appreciation of the contribution of iatrogenic factors, most notably: fluid overload, VALI from mechanical ventilation, transfusion of blood products and hospital acquired infection.138 While it is sobering to appreciate the negative role that healthcare systems have played, it has at least indicated the potential to prevent ARDS through simple quality improvement interventions.139 140 Similarly, while causes of ARDS that act directly on the lung, including pneumonia and gastric aspiration, are associated with a rapid progression to ARDS, indirect causes typified by severe sepsis commonly evolve into ARDS as part of a multiorgan dysfunction syndrome over 2–4 days.14
Scoring systems have been developed to predict progression to ARDS both in patients at risk and those with early lung injury. The Lung Injury Prediction Score (LIPS: table 13) is the product of a series of epidemiological studies.141 142 LIPS was designed to identify a population of patients at high risk of ARDS for prevention studies to be carried out by the National Institutes of Health’s Prevention and Early Treatment of Acute Lung Injury (PETAL) Network (http://petalnet.org/). LIPS-A was a large multicentre study to address the question of whether ARDS can be prevented with a drug, in this case aspirin, the latest in a succession of promising therapeutics for ARDS, which was supported by a plethora of positive preclinical data. Disappointingly, the study was negative and one contributing factor was that the score threshold for study inclusion produced only half the predicted number of ARDS cases, the study’s primary outcome.143 This raises concerns about the ability of LIPS to identify an enriched population of patients at risk for ARDS without the addition of factors such as biomarkers that can predict deterioration from at risk, to mild, to severe ARDS, and to death. Similarly, by characterising patients early in their clinical course before they develop ARDS, it has been possible to refine the parameters to the need for supplemental oxygen, an elevated respiratory rate and bilateral infiltrates on the chest radiograph to identify patients with early acute lung injury (EALI).144 Validation by means of a multicentre study prospectively evaluating the positive predictive value of a score comprising these variables would be required to generate a EALI score that could have a similar role to LIPS in future trials.
The lung injury prediction score
Secondary and tertiary prevention
Transfusion of blood products has been associated with the incidence of ARDS, nosocomial infection and mortality in critical illness. TRALI is defined as the onset of ARDS within 6 hours of the transfusion of any blood product in the absence of another risk factor.145 The most important mechanism of injury appears to be the interaction of preformed antibodies in the product with the host pulmonary vascular endothelium. Hence, products containing the most plasma confer the highest risk and the exclusion of female donors of products with high plasma volume has resulted in a decrease of roughly two-thirds in the incidence of TRALI. Transfusion of packed red cells using a threshold of 7 was non-inferior to a threshold of 9 g/dL and corresponding protocols restricting unnecessary transfusion should be introduced locally and practices audited.
There is a lack of evidence-based practices that decrease hospital acquired infection. An effective local antibiotic policy should aim to optimise antibiotic treatment according to local surveillance data and to ensure rapid de-escalation based on culture results. Recent evidence suggests that enteral nutrition is preferable to parenteral, and that underfeeding is less dangerous than overprovision. Finally, active rehabilitation, specialist outpatient follow-up and psychological support have been recommended for all survivors of severe critical illness in order to mitigate the associated neuropsychological effects and weakness.146
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- 145.↵
- 146.↵
Footnotes
Contributors Each author contributed elements to the final text.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests Each author contributed elements to the final text.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; internally peer reviewed.
Data sharing statement All data relevant to the study are included in the article or uploaded as supplementary information.