Article Text
Abstract
Introduction The prognosis of malignant pleural mesothelioma (MPM) is poor, with a median survival of 8–12 months. The ability to predict prognosis in MPM would help clinicians to make informed decisions regarding treatment and identify appropriate research opportunities for patients. The aims of this study were to examine associations between clinical and pathological information gathered during routine care, and prognosis of patients with MPM, and to develop a 6-month mortality risk prediction model.
Methods A retrospective cohort study of patients diagnosed with MPM at Queen Alexandra Hospital, Portsmouth, UK between December 2009 and September 2013. Multivariate analysis was performed on routinely available histological, clinical and laboratory data to assess the association between different factors and 6-month survival, with significant associations used to create a model to predict the risk of death within 6 months of diagnosis with MPM.
Results 100 patients were included in the analysis. Variables significantly associated with patient survival in multivariate analysis were age (HR 1.31, 95% CI 1.09 to 1.56), smoking status (current smoker HR 3.42, 95% CI 1.11 to 4.20), chest pain (HR 2.14, 95% CI 1.23 to 3.72), weight loss (HR 2.13, 95% CI 1.18 to 3.72), platelet count (HR 1.05, 95% CI 1.00 to 1.10), urea (HR 2.73, 95% CI 1.31 to 5.69) and adjusted calcium (HR 1.47, 95% CI 1.10 to 1.94). The resulting risk model had a c-statistic value of 0.76. A Hosmer-Lemeshow test confirmed good calibration of the model against the original dataset.
Conclusion Risk of death at 6 months in patients with a confirmed diagnosis of MPM can be predicted using variables readily available in clinical practice. The risk prediction model we have developed may be used to influence treatment decisions in patients with MPM. Further validation of the model requires evaluation of its performance on a separate dataset.
- lung cancer
- mesothelioma
- asbestos induced lung disease
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is the key question?
Is it possible to predict prognosis in patients with malignant pleural mesothelioma (MPM) using data collected routinely during patient care?
What is the bottom line?
In patients with a confirmed diagnosis of MPM, risk of death at 6 months can be predicted using variables readily available in clinical practice.
Why read on?
The risk prediction model we have developed could be used to influence treatment decisions in patients with MPM.
Introduction
Malignant pleural mesothelioma (MPM) is a rare cancer predominantly caused by asbestos which affects the pleura, a thin membrane of cells which lines the lungs and chest wall.1 The prognosis of MPM is generally regarded as poor, with a median survival of between 8 and 12 months.2 The UK currently has the highest death rate from MPM in the world,3 with approximately 2500 deaths per year.4 The ability to predict prognosis in MPM would help clinicians to make informed decisions regarding treatment, tailored to individual patients, such as early specialist palliative care.5 Predicting prognosis also enables clinicians to identify appropriate research opportunities for patients.
There exists a large body of evidence examining prognostic factors in MPM including numerous clinical factors such as basic epidemiological variables, clinical condition, imaging assessments and tumour features.6–9 Several prognostic scoring systems have been developed for MPM by combining prognostic variables, such as the European Organisation for Research and Treatment of Cancer6 and Cancer and Leukaemia Group B7 prognostic scoring systems, but these were derived from large series of patients from chemotherapy trials and may not reflect ‘real-world’ unselected patient cohorts. The LENT score is a prognostic scoring system for patients with malignant pleural effusions including mesothelioma10; however, this system is not specific for predicting survival in MPM and may not be applicable to patients with MPM without evidence of an effusion. More recently, a clinical prediction model for prognosis in MPM has been developed using survival at 18 months as the dependent variable11 using routinely available histological, clinical and laboratory information. However, since the median survival in patients with MPM is considered to be 8–12 months from diagnosis, identifying patients with an expected prognosis beyond 6 months would potentially be of greater value in guiding treatment decisions.
This study evaluated the Portsmouth city population, an area within close proximity of a dockyard with a historically large shipbuilding industry, where a significant amount of asbestos was used. As a result, the prevalence of asbestos-related respiratory disease including MPM among the local population is significantly greater than the UK average,12 13 with approximately 30 to 40 new cases of MPM diagnosed each year in Portsmouth. Optimal prognostication is therefore of particular importance in order to respond to the needs of the local population. The aim of this study was to identify clinical and pathological characteristics gathered during routine care, including demographic and clinical information, to predict risk of death in an unselected group of patients with MPM. These data were then studied for an association with outcomes and used to develop a mortality risk prediction model.
Methods
This was a retrospective cohort study of 100 consecutive patients aged over 18 years diagnosed with MPM at Queen Alexandra Hospital, Portsmouth, UK between December 2009 and September 2013. Data were collated from a pre-existing database, originally designed to evaluate the clinical services and patient pathway provided by the MPM service at Portsmouth. Identifiable patient information from the original database was anonymised for the purposes of the study. The dataset was censored for survival on 31 October 2014.
The outcome variable was patient survival time, measured as the time from the date of MPM diagnosis to the date of death. Potential predictor variables were chosen following a review of current literature,6–9 13–17 selected from data that are routinely available at the point of diagnosis including demographic characteristics and laboratory test results. These variables are listed in table 1. We did not adjust patient survival for treatment given the multitude of factors that influence treatment decisions, variation in treatment regimens and since the improvement in survival was thought to be modest.
Potential predictor variables for survival in patients diagnosed with malignant pleural mesothelioma in Portsmouth, UK
Statistical approach
A description of the study cohort for all potential risk factors was provided using mean and SD for continuous variables with a normal distribution, median and IQR for continuous variables with a skewed distribution, and frequency and percentage for categorical variables. As some patients in the study were still alive at time of data collection, it was necessary to analyse the data using survival analysis methods including Kaplan-Meier plots. All analyses were performed using Cox regression.
Univariate analysis was first performed to assess the association between each factor and survival. Where a linear relationship was not found to be appropriate, a non-linear relationship between the variable and the risk of death was assumed. The joint association between the variables and the outcome was then assessed in a multivariate analysis; only variables showing some evidence of an association with survival from the univariate analyses (p<0.1) were included. Variables with incomplete data were also excluded from the multivariate analysis.
The variables identified to be significant in the multivariable regression analysis were then used to create a risk model to predict the probability of death within 6 months of diagnosis for each patient. A 6-month cut-off was used, as it would allow for better treatment planning in a patient group where median survival is considered to be 8–12 months from diagnosis.
The linear combination of the coefficients was initially calculated. For categorical variables, the coefficient was added to the total if that factor was present. For continuous variables, the coefficient was multiplied by the value for that factor. Once the linear combination was calculated, a transformation was required to obtain the predicted probability.
The predicted probability was calculated using the following formulae:
y=Σ coefficients
p=1–0.99999944exp(y) where:
Σ=mathematical sum.
p=Predicted risk of death at 6 months.
exp=exponential function.
Two aspects of the model performance were then evaluated. First, the discrimination of the model was evaluated using Harrell’s C-statistic, broadly equivalent to the area under the ROC curve, examining the ability of the model to distinguish between high-risk and low-risk cases. Second, both the discrimination and calibration of the model was examined by dividing the predicted risk into three categories, each chosen to give a reasonably similar number of patients in each category. The observed percentage of graft loss within each of these three categories was calculated and this was compared with the predicted risk. The calibration of the model was also examined by the use of the Hosmer-Lemeshow test, comparing the observed and predicted number of events and non-events in the same categories.
Data were analysed using IBM SPSS statistical package V.22.0.
Ethical approval
As all patients in this study remained anonymous, we did not require individual consent in accordance with the Medical Research Council.18
Patient and public involvement
Opinion and feedback on study design was sought through the Hampshire Asbestos Support and Awareness Group (HASAG), a charity based in Hampshire, UK that supports people affected with MPM and their families. HASAG will also be involved in dissemination of the results via their newsletter and website.
Results
Data from a total of 100 consecutive patients diagnosed with MPM were included. Variables were collected within 2 weeks of initial presentation for all patients, apart from histological confirmation: histology was available for 92 patients at the time of diagnosis; two further patients received a cytological diagnosis; six patients received a radiological diagnosis, later confirmed on post mortem. The median time from first presentation to date of diagnosis was 14 days (IQR 8–50 days). Patient demographics and variables are summarised in table 2.
Demographics of 100 consecutive patients diagnosed with malignant pleural mesothelioma in Portsmouth, UK between December 2009 and September 2013
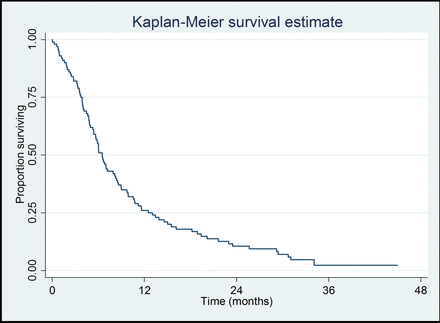
The primary outcome was patient survival: the 1-year survival was 26% (95% CI 18% to 35%). A graphical illustration of the survival times of the whole cohort is given in a Kaplan-Meier plot (figure 1).
Kaplan-Meier plot of survival times of all 100 patients with mesothelioma.
Univariate analysis
The association between each factor and survival was first examined in a series of univariate analyses (table 3). The size of the association between each predictor variable and survival was quantified using HRs, along with corresponding CIs.
Summary of the univariate analysis results for the association between the various prognostic factors and survival
When examined separately, a number of the variables were significantly associated with patient survival: age (HR 1.07, 95% CI 1.00 to 1.14), weight (HR 0.82, 95% CI 0.70 to 0.95), body mass index (BMI) (HR 0.71, 95% CI 0.52 to 0.96), smoking status (HR 2.20, 95% CI 1.07 to 4.51), performance status greater than 1 (HR 3.21, 95% CI 1.80 to 5.74), dyspnoea (HR 0.49, 95% CI 0.27 to 0.86), chest pain (HR 1.60, 95% CI 1.05 to 2.43), weight loss (HR 2.37, 95% CI 1.55 to 3.62), haemoglobin (HR 0.77, 95% CI 0.68 to 0.88), platelet count (HR 1.04, 95% CI 1.01 to 1.07), urea (HR 1.31, 95% CI 1.03 to 1.67), potassium (HR 2.21, 95% CI 1.09 to 4.49), creatinine (HR 40 042, 95% CI 9.2 to 1 768 852), albumin (HR 0.70, 95% CI 0.55 to 0.88), adjusted calcium (HR 1.37, 95% CI 1.10 to 1.70) and pleural fluid LDH (HR 4.26, 95% CI 1.66 to 11.0).
Multivariate analysis
The second stage of the analysis process examined how survival was affected by the variables jointly, in a multivariate analysis. As BMI and pleural fluid data were only available for 48 and 52 patients, respectively, these variables were omitted from the analysis. A backwards selection procedure was performed to retain only the independently statistically significant variables. The final model, based on data from 74 patients, is summarised in table 4.
Summary of the multivariate analysis results for the association between selected prognostic variables and survival
The multivariate results suggest that the predictor variables of age (HR 1.31, 95% CI 1.09 to 1.56), smoking status (current smoker HR 3.42, 95% CI 1.11 to 4.20), chest pain (HR 2.14, 95% CI 1.23 to 3.72), weight loss (HR 2.13, 95% CI 1.18 to 3.72), platelet count (HR 1.05, 95% CI 1.00 to 1.10), urea (HR 2.73, 95% CI 1.31 to 5.69) and adjusted calcium (HR 1.47, 95% CI 1.10 to 1.94) are independently associated with patient survival. After adjusting for the variables in the final model, there is no longer a significant effect of weight, performance status, dyspnoea, haemoglobin, potassium, creatinine or albumin on the outcome.
The univariate analysis suggests a non-linear relationship for age. However, after adjusting for the other factors, there was a steady increase in the risk of death with increased age: a 5-year increase in age was associated with a 31% increase in the risk of death.
Smoking status, while only of borderline significance in the univariate analysis, was found to be more strongly significant in the multivariate analysis. Ex-smokers and current smokers were at increased risk: the risk of death at any time was 3.4 times greater for current smokers and 2.16 times greater for ex-smokers than for non-smokers.
As in the univariate analyses, in the multivariate analysis patients with chest pain and weight loss were both at increased risk with a 2.14 and 2.13 times greater risk of death at any time, respectively. Higher urea and adjusted calcium values were also associated with increased risk.
The HRs for platelet count were broadly similar to those observed in the univariate analyses.
Development of a risk model to predict prognosis
Regression coefficients were then calculated for the risk model. These factors were used as they were found to be independently most significantly associated with patient survival. A summary of the regression coefficients for all variables in the final model are given in table 5.
Summary of the regression coefficients for chosen variables
Results of the risk model
The discrimination of the model was examined using Harrell’s c-statistic: a c-statistic value of 0.76 for this dataset suggests the model has some ability to discriminate between low-risk and high-risk patients. Patients were split into one of three groups based on their predicted risk of death within 6 months. A comparison of the predicted risk of death at 6 months and the actual observed occurrence of death at 6 months was made, and the results are summarised in table 6.
Comparison of the predicted risk of death at 6 months and actual observed occurrence of death at 6 months
The results demonstrate that the percentage of deaths predicted at 6 months increased proportionally with increased risk group, suggesting that the model has good discrimination. The actual percentage of observed deaths at 6 months was similar to the predicted values, demonstrating good calibration.
The Hosmer-Lemeshow test was also used to examine the calibration of the model in terms of the predicted cases of death within 6 months. A summary of the observed and predicted number of deaths is shown in table 7 along with the results of the test.
Summary of the observed and predicted number of deaths
The non-significant result suggests good agreement between observed and predicted numbers of cases of death at 6 months for each of the risk groups. Thus, this result suggests a good calibration of the model.
Discussion
This was a retrospective analysis of potential prognostic factors in patients newly diagnosed with MPM. These factors were then used to develop a risk prediction model for death at 6 months. We identified age, smoking status, symptoms of chest pain and weight loss at presentation, and raised platelet count, urea and creatinine, as markers of poor prognosis in MPM.
The demographics of our patient cohort broadly reflect those of patients with MPM diagnosed within the UK during this period,19 with a similar average age at diagnosis and strong male predominance. However, 1-year survival was lower than that seen nationally (26% vs 40%). Variation in survival between different centres has previously been recognised,5 and may be explained by performance status and comorbidity.
Clinical variables
The data from this study demonstrated a steady increase in the risk of death from MPM with increasing age. Age is widely accepted as a significant determinant of life expectancy. However, age in determining prognosis in patients in mesothelioma has been contentious. Although studies have previously reported age as a significant variable,20–22 there are studies that suggest age has no prognostic significance.8
This study also found that smoking status was of value in predicting risk of mortality at 6 months. The multivariate analysis showed smoking to be significantly associated with increased risk of death in both ex-smokers and current smokers. Previous studies have similarly not found a significant association between smoking status and prognosis in univariate analysis9 15; however, smoking status was consequently omitted from multivariate analysis in these studies. It is also possible that smoking status is a surrogate for other comorbid factors relating to prognosis which were not recorded elsewhere in the database.
Chest pain and weight loss were associated with poorer outcomes. Few studies have previously examined a direct correlation between baseline symptoms and prognosis in patients with MPM, with conflicting evidence in this area: studies have previously demonstrated no significant association between weight loss and survival,6 although one study has demonstrated an association between weight loss at diagnosis and survival at 18 months.11 Chest pain is likely to reflect tumour invasion and is a surrogate marker for cancer stage, whereas weight loss is a modifiable variable that can be directly addressed. A study examining quality of life in patients with MPM identified pain as a symptom significantly associated with worse global quality of life,22 and further work in this field has identified that baseline quality of life is a significant prognostic factor for survival.23 24 An American study evaluating the role of early specialist symptom control delivered by palliative care specialists in advanced lung cancer reported better quality of life and improved survival.25 This work has identified an as-yet unmet need for research investigating the benefits of better symptom control on improving survival in mesothelioma.
Surprisingly, variables such as performance status, histological subtype and patient gender which in previous literature have been associated with prognostic outcome14 21 26 27 were not found to be significant. It is possible that in some cases, patients with suspected MPM and worse performance states may not have been included in the database as their frailty may have limited the ability to confirm diagnosis of MPM. This may explain why performance status, which is generally accepted as a strong predictor of mortality, was not significantly associated with risk of death in this study. Similarly, since some histological subtypes have previously been associated with rapid decline in performance status, it is possible that frailty in patients with more aggressive histological subtypes limited the ability to confirm a diagnosis of MPM and thus were not included in the database. While reflective of the demographic of patients diagnosed with MPM, it is possible the small size of the female cohort influenced the strength of the association with gender.
Biochemical variables
Certain routine biochemical markers act as a surrogate for the patient’s overall condition, as a reflection of systemic illness. Studies have previously demonstrated the value of routine laboratory tests in predicting mortality in other pulmonary diseases, such as chronic obstructive pulmonary disease.28 However, to date there has been limited data available on routinely available biochemical variables in mesothelioma.
Previous work has showed certain cancer types including mesothelioma to produce large amounts of interleukin-6 (IL-6)29 30 which are strongly correlated with platelet count, suggesting that IL-6 may have a role in tumour-associated thrombocytosis.29 Data on platelet count as a predictor of survival are conflicting, with some evidence showing no prognostic relevance.31 However, two more recent studies showed a significant association with higher platelet counts and poorer survival15 16; this was also demonstrated in our study, although the effect in the multivariate analysis was small.
The multivariate analysis identified elevated serum urea and calcium to be associated with an increased risk of death at 6 months. While there are limited data on urea as a prognostic marker for mesothelioma, a recent retrospective analysis of 114 patients also identified elevated serum urea to be a predictor of poor outcome17 which is supportive of our findings.
A limitation to this work is that, as with many other studies evaluating prognosis, analysis was tested on a retrospective population, thus limiting the quality of evidence. Another shortcoming was the proportion of missing data encountered for certain variables, attributed to the retrospective nature of the study, resulting in their exclusion from analysis; for example, BMI and pleural fluid data.
Conclusion
In summary, we have developed a model to predict risk of death at 6 months in patients with a confirmed diagnosis of MPM using variables readily available in clinical practice, derived from an unselected population at diagnosis. Although these factors alone cannot be used in isolation to predict prognosis, their presence may provide clinical teams with additional information when discussing life expectancy with patients and their families. Further validation of the model requires evaluation of its performance on a separate dataset, which we intend to do with data from a recently completed national study (RESPECT-Meso7).
References
Footnotes
Contributors SaG, CF, PB, ShG, SE and AC contributed to the study design. SaG, DN, TJ, SB, SK, LM, ShG, SE, RS and LB contributed to the data collection. PB conducted the statistical analysis. SaG, DL, CF and AC drafted the initial manuscript. SaG, DL, PB and AC revised the manuscript. All authors approved the final manuscript as submitted.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication Not required.
Ethics approval Ethical approval for this research was granted on 16 April 2014 by the National Research Ethics Service (NRES) Committee London–Camberwell St Giles Research Ethics Committee (REC) (reference no. 14/LO/0628).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request, by contacting the Portsmouth Hospital Respiratory Research department (respiratoryresearch@porthosp.nhs.uk).