Article Text
Abstract
Introduction Chronic obstructive pulmonary disease (COPD) is characterised by exacerbations of respiratory disease, frequently requiring hospital admission. Pulmonary rehabilitation can reduce the likelihood of future hospitalisation, but programme uptake is poor. This study aims to compare hospital readmission rates, clinical outcomes and costs between people with COPD who undertake a home-based programme of pulmonary rehabilitation commenced early (within 2 weeks) of hospital discharge with usual care.
Methods A multisite randomised controlled trial, powered for superiority, will be conducted in Australia. Eligible patients admitted to one of the participating sites for an exacerbation of COPD will be invited to participate. Participants will be randomised 1:1. Intervention group participants will undertake an 8-week programme of home-based pulmonary rehabilitation commencing within 2 weeks of hospital discharge. Control group participants will receive usual care and a weekly phone call for attention control. Outcomes will be measured by a blinded assessor at baseline, after the intervention (week 9–10 posthospital discharge), and at 12 months follow-up. The primary outcome is hospital readmission at 12 months follow-up.
Ethics and dissemination Human Research Ethics approval for all sites provided by Alfred Health (Project 51216). Findings will be published in peer-reviewed journals, conferences and lay publications.
Trial registration number ACTRN12619001122145.
- COPD exacerbations
- exercise
- pulmonary rehabilitation
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
People with chronic obstructive pulmonary disease (COPD) experience a progressive decline in lung function, reduced exercise tolerance, marked breathlessness and multiple comorbidities.1 Recurrent bouts of acute deterioration in symptoms are common in COPD, and such exacerbations2 commonly require hospital admission for treatment and management. Hospitalisation for an exacerbation of COPD is associated with increased morbidity, readmission, resource utilisation and mortality.3 Additionally, COPD exacerbations reduce both quality of life and physical function, which may not spontaneously recover.1 3 For people with COPD avoiding exacerbations, and resultant hospitalisations are outcomes of key importance.4
Pulmonary rehabilitation, a comprehensive programme including exercise training and self-management education,5 is an established management strategy for people with COPD recommended in guidelines.6 7 Systematic reviews with meta-analyses and large cohort studies have both demonstrated that completion of pulmonary rehabilitation reduces future exacerbations, need for hospitalisation and hospital length of stay.8–10 The reduced likelihood of hospital admission is particularly evident when pulmonary rehabilitation is undertaken following an exacerbation (pooled OR 0.44, 95% CI 0.21 to 0.91).1 However, following hospitalisation for an exacerbation, fewer than 10% of people with COPD are referred to outpatient pulmonary rehabilitation on hospital discharge11 and fewer than 3% attend outpatient pulmonary rehabilitation in the year following hospital discharge.12 Low rates of pulmonary rehabilitation referral are contributed to by limited knowledge and experience of pulmonary rehabilitation by healthcare professionals.13 14 Poor referral rates are further compounded by key patient-related barriers to attendance at outpatient pulmonary rehabilitation programmes including limited understanding of programme requirements and benefits, and difficulties associated with travel and transport.14 15
Despite evidence and growing interest in pulmonary rehabilitation following an exacerbation,16 previous studies have had challenges in terms of participant recruitment and retention,17 18 had variable clinical efficacy,11 19–23 and been heterogeneous in regard to timing of rehabilitation commencement, training duration and intensity and length of follow-up period.1 When to commence pulmonary rehabilitation following an exacerbation remains a key issue. A randomised controlled trial (RCT) with 320 participants hospitalised for an exacerbation of COPD who were allocated to either very early rehabilitation (commenced within 48 hours of admission) or to usual care reported significantly increased mortality at 1 year in the very early rehabilitation group (OR 1.74, 95% CI 1.05 to 2.88).19 The reasons for increased mortality are not known, but a meta-analysis of rehabilitation interventions post exacerbation suggests the timing of intervention delivery is crucial, with pooled data for studies that commenced pulmonary rehabilitation in the (very) early inpatient period demonstrating increased odds of death (OR 1.74, 95% CI 1.07 to 2.84), when compared with those studies that waited to commence rehabilitation in the period following hospital discharge (OR 0.25, 95% CI 0.08 to 0.75).24 A rehabilitation delivery model that is acceptable to patients in the period following hospital discharge, efficacious and amenable to implementation in clinical practice is still to be elucidated.
Alternative models of pulmonary rehabilitation delivery that overcome patient barriers to attendance at outpatient programmes, have the potential to improve rates of rehabilitation completion following an exacerbation and reduce the need for subsequent hospitalisation. A recent UK national audit reported significant reductions in hospital admissions and length of stay for people who complete pulmonary rehabilitation vs non-completers (admissions: 13% vs 27%; length of stay: 3 days vs 7.2 days; both p<0.001).25 Previously, we have demonstrated higher programme completion rates with a home-based model of pulmonary rehabilitation when compared with traditional outpatient pulmonary rehabilitation in a randomised controlled equivalence trial in people with stable COPD (91% vs 49% completion).26 Equivalent clinical outcomes, at similar costs, were also achieved.26 In a small feasibility study the same home-based model of pulmonary rehabilitation was found to be satisfactory to people with COPD following hospitalisation for an exacerbation and achieved clinically meaningful improvement in quality of life and functional exercise capacity.27 Although programme uptake was modest, 80% of participants who commenced the home-based pulmonary rehabilitation programme went on to complete.27 Whether this translates into reduced need for future hospitalisation is unknown.
The aims of this study are to compare a home-based programme of pulmonary rehabilitation, delivered by telephone and commenced within 2 weeks after hospital discharge, to usual care, in people with COPD following hospitalisation for an exacerbation. In particular we aim to determine: (1) hospital readmission rates; (2) clinical outcomes and (3) costs. We hypothesise that, when compared with usual care, a home-based programme of pulmonary rehabilitation commenced early (within 2 weeks) following hospital discharge for an exacerbation of COPD will: (1) reduce hospital admissions; (2) produce clinically meaningful improvements in symptoms, health-related quality of life and exercise capacity which are greater than those seen in usual care and (3) be more cost-effective, from a societal perspective.
Methods and analysis
Design
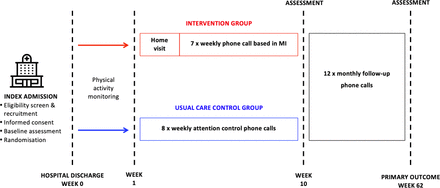
A randomised, controlled, assessor-blinded trial, powered for superiority, will be conducted at three metropolitan and two regional centres, across multiple states, in Australia. The trial was registered prospectively at www.anzctr.org.au (ACTRN 12619001122145) on August 12 2019, including details of trial sites. Study procedures are illustrated in figure 1.
Study procedures. MI, motivational interviewing.
Participants
Individuals admitted to hospital for an exacerbation of COPD at one of the participating sites will be invited to participate. Inclusion and exclusion criteria are detailed in table 1.
Inclusion and exclusion criteria
A clinician or researcher will provide written and verbal information to all eligible participants during their hospital stay. Visual information using a video is also available. Written informed consent will be provided by all individuals who agree to participate. Trial participation will not alter routine COPD management, nor management of any other health condition.
Recruitment and randomisation
Following informed consent, participants will be randomly allocated (1:1) to either the intervention (home-based pulmonary rehabilitation commencing early (within 2 weeks) following hospital discharge, ie, early HomeBase), or to a control group. An independent, external, service (https://randomisation.griffith.edu.au/) will be used to generate and conceal the randomisation sequence from investigators. The randomisation scheme will be computer generated, using permuted blocks and stratified for (1) site of recruitment; as well as (2) disease severity (forced expiratory volume in 1 s (FEV1) ≥50%predicted and <50%predicted) and (3) age (≥75 vs<75 years)—noting both disease and age are powerful predictors of future hospitalisation and mortality in COPD.28 Randomisation will take place following completion of the baseline assessment. Due to the nature of the intervention (exercise rehabilitation) it will not be possible to blind participants to the intervention, however, all outcome measures will be collected by an independent assessor blind to group allocation. Participant flow through the trial will be reported according to the recommendations of the Consolidated Standard of Reporting Trials (CONSORT and CONSERVE-CONSORT).29 30
Interventions
Usual care
All participants will receive usual care, including medical management for their exacerbation based on COPD-X guidelines6 (medications as indicated, controlled oxygen therapy and ventilatory assistance as required) and physiotherapy, commencing on the first day of admission.
Control group
In addition to usual care, participants randomised to the control group will receive a weekly telephone call for the period of the intervention (8 weeks). Telephone calls will not include any health information or advice on physical activity or exercise and are designed to serve as attention control. A standardised telephone call record sheet will be used to record participant comments in response to how they are feeling and whether they have visited the doctor, hospital or emergency department in the previous week. In line with guideline recommendations for management of an acute exacerbation of COPD,6 participants in the control group may be referred to traditional outpatient (centre-based) pulmonary rehabilitation after discharge from hospital. The number of control participants who take up such a referral will be documented.
Intervention group: home-based pulmonary rehabilitation commenced early after hospitalisation (early HomeBase)
Alongside usual care, participants in the intervention group will undertake an 8-week home-based, pulmonary rehabilitation programme commenced within 2 weeks of hospital discharge. Early home-based pulmonary rehabilitation will be delivered according to our previously published protocol in stable COPD.26 The nature of the home-based intervention, that is, being delivered by telephone, conforms with the definitions of both telehealth and telerehabilitation being healthcare (specifically rehabilitation) provided at a distance through the use of telecommunication (or virtual) technology.31
Home visit
According to our previously published model,26 the programme will commence with one home-visit by a physiotherapist to establish exercise goals and safety, and supervise the first exercise session. Also included in the home-visit will be components of the self-management programme that cannot be adequately covered by subsequent telephone calls (eg, review of inhaler technique),26
Self-monitored exercise training
Participants will undertake an aerobic and resistance training programme, with intensity of training individualised according to symptoms to achieve a Borg-CR10 breathlessness rating of 3–4 with exercise.32 For aerobic training, a walking distance goal will be set, with distance assessed using pedometers. Where participants have interest and access to alternative aerobic training modalities, for example, cycling or swimming, these may undertaken in addition to or instead of walking training. The target will be 30 min of aerobic exercise per day, which may be undertaken in multiple shorter periods. At least five sessions of aerobic training per week, will be encouraged. Resistance training will use functional activities and equipment that are readily accessible in the home environment, such as sit-to-stand from a dining chair and water bottles for upper limb weights. Participants will record details of their exercise training in a home exercise diary.
The initial home-visit will be followed by seven once-weekly telephone calls from a pulmonary rehabilitation clinician, using a motivational interviewing approach. Clinicians delivering intervention phone calls will undertake specialised motivational interviewing training, and receive ongoing support and training from an expert motivational interviewing practitioner, to ensure competency. During the telephone calls the pulmonary rehabilitation clinician will (1) review symptoms and the home diary; (2) progress the exercise prescription through discussion of exercise goals, with goals recorded in the home diary and (3) facilitate self management of exacerbations and deliver self-management education via scripted telephone modules. These previously developed,26 structured telephone modules will be used to explore and build motivation for exercise participation, then move towards commitment and action.33
Self-management education
To address international guidelines,7 participants will be provided with a selection of topics related to COPD self-care and encouraged to choose a topic of relevance to them for discussion each week during their telephone call with the clinician.34 Education on how to manage an acute exacerbation of COPD and the importance of long-term exercise maintenance will be a focus of self-management training for all participants. The aim is to improve self-efficacy by increasing the knowledge and skills required to optimally manage COPD. This approach requires goal setting, problem solving, decision making and taking action based on a predefined plan.34 Other positive adaptive behaviours that may be facilitated during self-management training include medication adherence, smoking cessation and changing nutritional habits. Health goals will be documented in the home exercise diary and reviewed weekly. Participants will also receive self-management education resources from Lung Foundation Australia. These resources have been developed to enable people with COPD to undertake the educational component of pulmonary rehabilitation from the comfort of their own home and include a book (Better Living with COPD: A Patient Guide(third Ed))35 and access to the C.O.P.E (COPD Online Patient Education) programme.
Ongoing exercise participation
At the completion of the rehabilitation period participants in the intervention group will be offered the opportunity to join a supervised exercise maintenance programme to promote ongoing exercise adherence, in line with national standards for postpulmonary rehabilitation care.36 Acceptance of a referral will be documented. Participants in the intervention group will be precluded from attending standard outpatient pulmonary rehabilitation only during the intervention period (ie, weeks 0–10).
Safety
Prior to commencing each unsupervised exercise session at home, intervention group participants will be asked to complete a checklist to ensure clinical stability for exercise (see online supplemental material). If the participant has symptoms of an exacerbation, the participant will be instructed to refrain from exercise and contact one of the researchers. If discussion confirms symptoms are likely to be indicative of a moderate exacerbation then the participant will be instructed not to exercise and to seek medical attention. If there is evidence of a severe exacerbation, an ambulance will be called. This is consistent with procedures that would be undertaken in a centre-based pulmonary rehabilitation programme and were successfully used previously.26
Supplemental material
To further monitor for safety, all participants (intervention and control groups) will be asked about emergency department presentation and/or hospital admission at their weekly phone call during the intervention period, and during their monthly phone call during the 12-month follow-up period. In the event a participant is unable to be reached by telephone for a scheduled call, a phone-call will be made to the next-of-kin to check on participant well-being.
Outcomes
Baseline participant characteristics including age, sex, lung function, frailty score,37 medications and hospitalisations will be collected from the participants and the medical record during the index hospital admission. Clinical outcome measures (table 2) will be assessed at baseline, at the end of the intervention (week 9–10 posthospital discharge), and at 12 months follow-up. All outcome measures will be collected by a researcher blind to group allocation. Participants will be asked to attend the hospital of recruitment for the end-intervention and follow-up assessments to standardise the administration of assessments across groups. The proportion of participants in both groups who take up an offer of centre-based pulmonary rehabilitation at any time in the study period will be recorded. The number of rehabilitation sessions attended (intervention and/or follow-up period) will be documented, with programme completion defined a priori as undertaking a minimum of 70% of planned sessions in any one course of pulmonary rehabilitation.38
Clinical outcome measures and assessment time points
The primary outcome is hospital readmission at 12 months. Readmission to hospital is a powerful predictor of poor outcomes in people with COPD.39 Hospital readmissions within the 12-month follow-up period will be determined using hospital medical records and cross-checked with patient self-report via monthly telephone calls.26 40
Economic evaluation
Economic evaluation will take a societal perspective,41 and include an incremental cost-effective analysis, as described previously.40 Costs incurred by all stakeholders, including out-of-pocket costs to participants for healthcare services and the cost of delivering the intervention, will be included in the evaluation. Healthcare utilisation by participants, including visits to health professionals and any hospitalisations, will be recorded in a diary for 12 months following the intervention period. Participants will also be telephoned monthly for 12 months to facilitate diary completion and to capture patient-reported information on hospitalisation episodes. Hospitalisation and use of other hospital services will be confirmed by medical record audit at the completion of 12 months follow-up. Health benefits in terms of quality-adjusted life-years will be estimated from the utility index calculated by applying a social tariff to the EQ-5D-5L42 and compared between groups. A separate assessment of cost-effectiveness will be undertaken by comparing the number of hospital admissions per participant in the 12-month follow-up period to the incremental cost of averting an additional hospital admission.
Statistical analysis
Sample size
To detect a difference in hospital readmission between the intervention group (early home-based pulmonary rehabilitation) and control group at 12 months follow-up, a total of 132 participants (66 participants in each group) will be required to detect a reduction in readmission rate to 30% with 80% power and a significance level of p<0.05. This assumes a rate of readmission of 57%22 in the control group. An additional 34 participants will be recruited to allow for 20% drop-out. This is a conservative estimate for attrition, with our previous studies of home-based pulmonary rehabilitation demonstrating around 15% drop-out rate26; we will, therefore, randomise a total of 166 participants.
Data analysis plan
Analysis will use intention-to-treat principles, with inclusion of all randomised participants regardless of programme completion. Protocol violations will not constitute grounds for withdrawal. A participant will be considered to have withdrawn from the study if consent is revoked. Where this occurs, no further assessments will be performed, however, participants will be informed that unless permission is expressly denied data collected up to the time of withdrawal will form part of the study results. Withdrawn participants will not be replaced. For the primary hypothesis (hospital readmission), the relative risk in the intervention group compared with the usual care control group will be reported with a 95% CI. Secondary continuous outcomes will be examined using linear mixed models with fixed effects for group, time and group × time interaction, in addition to disease severity and age. Site will be included in the model as a random effect. A per protocol analysis will be undertaken including those who complete the home-based pulmonary programme (≥70% of sessions attended). The proportion of participants in both groups who attend at least 70% of a pulmonary rehabilitation programme (home-based pulmonary rehabilitation intervention and/or outpatient pulmonary rehabilitation) in the study period (from hospital discharge to 12 months follow-up) will be compared using logistic regression. We will explore time to readmission using Cox regression, controlling for programme completion.
Data management
A purpose-built on-line database (www.adeptrs.com), with encryption and password protection, will be used to store electronic data. No identifying information will be stored in the online database. Hard copy original data collection forms will be stored in a locked filing cabinet within a locked office at the site of participant recruitment.
Monitoring
A multidisciplinary data safety and monitoring committee who, collectively, have experience in the management of patients with COPD, the delivery of pulmonary rehabilitation and the conduct and monitoring of RCTs, will meet a minimum of twice yearly. The Committee will include a biostatistician, and be chaired by a respiratory physician who is independent of the study team and the trial sites. The data safety monitoring committee will review data in a blinded fashion.
Dissemination
It is intended study findings will be disseminated via publication in peer-reviewed journals and presentation at conferences. A plain-English summary will be sent to participants at the conclusion of the trial, and results conveyed to people with COPD through lay publications and seminars.
Discussion
Pulmonary rehabilitation is a proven treatment for people with COPD8 yet is widely underused, particularly following an episode of hospitalisation for an exacerbation.11 Despite guideline recommendations for referral to pulmonary rehabilitation following exacerbation,7 there is a widespread lack of implementation of outpatient pulmonary rehabilitation after hospitalisation. In addition, doubts around timing of commencement of rehabilitation programmes following exacerbation have led to international calls for research evaluating alternative models of pulmonary rehabilitation that offer flexibility in terms of setting, type of training and timing of intervention delivery following hospitalisation.43 44
COPD is the most prevalent respiratory disease worldwide45 and is expected to become the leading cause of mortality, globally, within the next two decades.46 The economic burden of COPD is considerable, at both a patient and health-system level,47 with nearly three-quarters of healthcare costs associated with COPD attributable to exacerbations requiring hospitalisation.48 Pulmonary rehabilitation is a relatively low-cost, cost-effective treatment strategy49 that can reduce the likelihood of future need for hospitalisation due to exacerbation.8 To impact on rehospitalisation rates more people with COPD need to complete pulmonary rehabilitation.25 The period early posthospitalisation appears critical to reducing the likelihood of future hospitalisation, however key barriers to attending outpatient pulmonary rehabilitation must be overcome in order to achieve this outcome. The American Thoracic Society/European Respiratory Society Pulmonary Rehabilitation Policy Statement has identified as key research priorities the investigation of models of pulmonary rehabilitation delivery that improve access and uptake for patients; establish the cost-effectiveness of programmes, and address patient barriers to programme access, enrolment and completion.43 The randomised, controlled, superiority, trial described here, with embedded economic analysis, uses an alternative (home-based) model of pulmonary rehabilitation delivery, rigorously tested in stable patients,26 to address these research priorities and outcomes of importance to patients and the health system.
If this study demonstrates that home-based pulmonary rehabilitation, commenced within 2 weeks of hospital discharge, is effective at reducing hospital admissions and improving rehabilitation completion rates it will offer an accessible model of pulmonary rehabilitation, requiring minimal resources, for the period following COPD exacerbation. This low-cost home-based model of pulmonary rehabilitation is designed for rapid uptake into clinical practice on a global scale, including in locations where access is particularly limited, such as low-resource settings.
Patient and public involvement
The experiences of patients from our previous trials of home-based pulmonary rehabilitation26 and our pilot study of early home-based pulmonary rehabilitation27 informed the development of the protocol including timing of recruitment and assessments and intervention resources. People living with chronic lung disease provided patient review of intervention resources through the Lung Foundation Australia patient advocacy groups. Hospitalisation was selected as the primary outcome of the trial as it is an outcome of key importance to people with COPD.4
Trial status
Recruitment to the trial commenced at a single site (Alfred Health, Melbourne) in January 2020, with the remaining sites scheduled to commence recruitment by April 20, 2020. Due to delays associated with the COVID-19 pandemic, recruitment at all sites had commenced by November 2020. It is estimated the recruitment goal will be met by December 2023.
Ethics statements
Patient consent for publication
Ethics approval
Human Research Ethics approval was provided by Alfred Health for all sites (Project ID 51216, Local reference 142/19) with local governance approvals obtained from all participating sites.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Procured funding: NSC, AEH, CFM, AM, PO’H and GH. Conceptualisation and design: NSC, AEH, CFM, AM, PO’H and GH. Drafting protocol manuscript: NSC and AEH. Critical review of protocol manuscript: AEH, CFM, AM, PO’H, GH, AL, LS, RJM, JB, HM, SG, ATB, CLM, CR, EW, AN, L-LT, NL, SvH, MS, HC, MB, BW, HB, JB, TM, MC, CB and JM.
Funding This work is supported by a National Health and Medical Research Council (NHMRC) project grant (GNT1157313) held by NS Cox and AE Holland.
Competing interests NSC and AEH: declare grant funding from the NHMRC paid to their institution for the conduct of the trial. CFM declares board roles as Chair COPD National Program, Lung Foundation Australia; and Medical Director Institute for Breathing and Sleep. For all other authors, no competing interests are declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.