Abstract
Objectives
To evaluate effects of meals in difficult-to-wean tracheostomised patients with chronic obstructive pulmonary diseases during spontaneous breathing or Inspiratory Pressure Support.
Design
Prospective, crossover, randomised, and physiological study.
Setting
Weaning centre.
Patients
Sixteen COPD undergoing either decreasing levels of pressure support or increasing periods of spontaneous breathing.
Measurements
Each patient underwent monitoring during a 30-min procedure, during and after meals either under pressure support or spontaneous breathing on two consecutive days. Inductance plethysmography was used to monitor respiratory rate and tidal volume. Tidal volume by a flow transducer, arterial oxygen saturation, pulse rate, end-tidal CO2, and dyspnoea by a visual analogue scale were also assessed.
Results
ANOVA analysis showed a significant increase under spontaneous breathing for respiratory rate (P<0.001) and for end tidal CO2 (P<0.03) induced by the meals. Inspiratory pressure support was associated to significantly greater tidal volume (P<0.001), lower respiratory rate (P<0.032), lower respiratory rate/tidal volume (P<0.001), and lower pulse rate (P<0.047) than spontaneous breathing. Under spontaneous breathing but not under pressure support a statistically worsening in meal-induced dispnoea (P<0.001) was found.
Conclusions
In tracheostomised difficult-to-wean COPD patients: 1) under unassisted breathing, meals may induce an increase in respiratory rate, end-tidal CO2, and dyspnoea; 2) inspiratory pressure support ventilation prevents dyspnoea from worsening during meals.
Similar content being viewed by others
Introduction
Among the activities of daily life, meals represent an invaluable event for all people. The co-ordination of eating, drinking, and breathing in seven healthy adults has been reported [1]. Although breathing became more irregular during this activity no change in tidal volume, inspiratory and expiratory duration or minute ventilation was found [1].
Patients with moderate-to-severe chronic disease such as post-acute stroke [2] or children with severe cerebral palsy [3] may present hypoxaemia during eating. It is has been speculated that meal-related episodes of desaturation may occur as a result of aspiration of fluid/food into the airway which may eventually cause a mismatch of pulmonary ventilation and perfusion and, ultimately, hypoxaemia [2, 4]. In patients recovering from a stroke other authors assumed as a cause of meal-related desaturation an incoordination of breathing [5, 6]. Physiological monitoring during eating might provide an early warning, which in turn would prevent or reduce serious complications [7].
Studies have evaluated arterial oxygen saturation, arterial oxygen tension or oxygen uptake during daily activities in patients with chronic obstructive pulmonary disease (COPD) [8, 9, 10, 11, 12] showing irregular breathing and desaturation during walking, washing, eating [10], defecation [11], and a high oxygen uptake during simple activities involving the upper limbs [12]. In most of these patients, the extent of desaturation during meals was relatively small and of negligible haemodynamic consequence [10]. Several factors have been suggested to explain meal-related oxygen desaturations: gastrointestinal disorders [13], psychosocial factors [13], inadequate dietary intake for energy expenditure [14], decrease in minute ventilation as a result of interrupted breathing while chewing and swallowing [14], alteration in ventilation to perfusion relationships [14].
Weaning from artificial nutritional intake with the subsequent possibility to eat assumes an essential rehabilitative outcome in severe tracheostomised difficult-to-wean COPD patients [15] who may undergo different weaning protocols such as decreasing levels of inspiratory pressure support or increasing periods of spontaneous breathing [16]. Preliminary results of the study reported in this paper have been published as abstract [17] and have suggested the occurrence of modification in breathing pattern, heart rate, and dispnoea during meal-times in tracheostomised patients. Nevertheless, there is lack of information on the physiological effects of meals in these patients, not only in spontaneous breathing but also during assisted ventilation. Therefore, the aim of this study was to evaluate both physiological effects and subjective sensations induced by meals either in spontaneous breathing or in pressure support in tracheostomised difficult-to-wean COPD patients.
Methods
The investigative protocol was approved by the Institutional Ethic Committee of the S. Maugeri Foundation, Italy and was conducted according to the declaration of Helsinki. Informed consent was obtained from all the patients before enrolment into the study.
Patients
Sixteen tracheostomised difficult-to-wean COPD patients (cannula size 7.5 mm ID to 8 mm ID) undergone mechanical ventilation for at least 15 days, admitted to the weaning centre (WC) of Gussago from 30 October 1998 to 30 September 2004 were studied. All patients were known to be affected with COPD according to the American Thoracic Society criteria [18], or with high probability of the disease based on the clinical history, physical examination, chest X-ray and, when available, previous pulmonary function tests in stable state. In their stable state, most of patients were on long-term oxygen therapy and all were taking standard medical therapy, including beta 2-agonists, anti-cholinergic agents, and diuretics, if needed. These patients had been transferred to our WC from Intensive Care Units (ICUs) of other Hospitals because the caring physicians classified them as difficult-to-wean after some weaning attempts failed and tracheotomy was performed.
At the time of their entry into the study all the patients were ventilated (Evita 2 Draeger, Moislinger, Germany) with Pressure Support Ventilation (15±2 cmH2O) adjusted to achieve pH>7.35, SatO2 >92% at inspiratory oxygen fraction (FiO2) ≤ 0.4, respiratory rate ≥ 10 breaths.min-1 and ≤ 25 breaths.min-1. An external positive end-expiratory pressure <6 cmH2O (3.8±1.1 cmH2O) was added when intrinsic PEEP (PEEPi) was suspected on clinical basis. Medical therapy was optimised and patients’ airway secretions were frequently suctioned. In our WC, patients underwent one of the following weaning protocols: twice a day, the physician decreased the level of pressure support or increased periods of spontaneous breathing disconnecting patients from the ventilator according to modalities which have been previously reported [16].
Patients with concomitant neurological diseases unrelated to hypercapnic encephalopathy, other respiratory diseases, cancer and other severe systemic diseases, haemodynamic instability, lung or systemic infections, dysphagia or inability to swallow while sitting on an armchair were excluded from the study.
Measurements
The following data were recorded:
-
Anthropometrics and clinical conditions: age, body mass index, resting energy expenditure, temperature, use of steroids at the study time, causes of relapse before admission in the ICU, ICU length of stay before and APACHE II score at WC admission, days of tracheotomy, and outcome after discharge from the WC.
-
The outpatient clinic records obtained lung function data in the last period of stability before ICU admission. When these data were not available dynamic volumes (forced expiratory volume at first second-FEV1% pred., and forced vital capacity—FVC % pred.) were assessed by means of a portable spirometer at discharge with tracheostomised patients breathing through a mouthpiece having their fenestrated cannula closed by means of a cap.
-
Arterial blood gases were measured by means of an automated analyser on blood samples from the radial artery with patients under assisted ventilation at a FiO2 able to maintain SaTO2>92%.
-
Breathing pattern (respiratory rate, tidal volume, and minute ventilation), were assessed by means of respiratory inductance plethysmography (Respitrace Plus; ambulatory monitoring Ardsley, N.Y., USA). Quantitative diagnostic calibration [19] was done immediately before the study, calibrating the sum of rib cage and abdominal signals against the signal of tidal volume obtained by a flow transducer (Bicore, Irvine Calif., USA) with patients in sitting position breathing spontaneously through their usual cuffed tracheotomy cannula. The ratio between respiratory rate and tidal volume (ml) *1,000 (RR/VT) was calculated as the index of rapid shallow breathing [20]. The plethysmography monitoring used for the entire study allowed us to average respiratory and heart rate data for each 30-min period.
-
Arterial oxygen saturation and pulse rate were monitored by means of a pulse oximeter (Propac; Dragerwerk, Lubeck, Germany). The information continuously recorded for pulse oxymetry have automatically measured the variability of the two parameters during the whole study period: the data used for statistical analysis were the mean recording for each period of study (before, during, and after meals).
-
End-tidal CO2 by means of a capnometry (Lifescope P, Nihon Kohden) connected to the tracheotomy cannula. The data measured and showed on the screen monitor were recorded baseline, at the end of the meals, and at the end of the recovery time: the same data were used for the statistical analysis.
-
Dyspnoea was assessed by means of a visual analogue scale (VAS) [21] and recorded by a nurse not involved in the study. VAS consisted of a 20-cm horizontal line (0 = no dyspnoea; 20 maximal dyspnoea). The data have been presented in the text and in the figures as percentage of the maximum value.
-
Need of oxygen supply (l/min or FiO2) and number of suctions performed during the study were also recorded.
-
Resting energy expenditure was calculated by means of the Harris and Benedict formula and corrected for a stress index of 1.5 according to a moderate stress due to prolonged ventilation condition to obtain the prescription of daily calories; this one was the same in the two days of study (ranging 340–800 kcal). The distribution of calories intake was as follows: percentage of proteins (40%), glucides (30%) and lipids (30%). To avoid differences among patients and between the 2 days of study, the availability of meal-times was 30 min in every case while patients were eating without help. If patients had finished meals before the scheduled time they would have been submitted to the 30-min recording of recovery time: in contrast, if patients did not finish the meals within 30 min the meals would be stopped and the total intake of calories would be measured. The actual calory intake was recorded by a dietician at the end of each meal.
Protocol
From an ethical point of view we could not prevent the possibility of aspirating our patients during meals. To reduce the possibility that suctions might influence the results with a possible decrease in airway resistance, we performed suction in every patient at least 10 min before starting the meal. The possible effect of suction on physiological variables in patients in whom it was necessary was diminished in the 30 min of continuous recording of data.
In every case, 30 min before the study, an inhaled short-acting beta agonist was administered through the tracheotomy cannula. The possible improvement of dyspnoea after prescription of beta 2 stimulants is well known [22]: consequently we prescribed this drug to all patients before the study, to avoid differences among patients in bronchodilatation effects.
Respiratory rate, tidal volume, end-tidal CO2, SatO2, and pulse rate were continuously monitored for 30 min before (T030m), during (T130m), and until 30 min after the meals (T230m). To decrease the variability of the response, breathing pattern, SatO2 and pulse rate data averaged for the whole 30 min periods of the study. Dyspnoea was assessed immediately before the start of (T0), at the end of (T1), and 30 min after meals (T2).
Assessments were performed under the two conditions (assisted and unassisted ventilation) randomly applied on two consecutive days. Independent of modality of long-term weaning (either decreasing levels of pressure support or increasing periods of spontaneous breathing) the level of pressure support applied during the study was that used the day before. In either condition FiO2 was adjusted in order to maintain SatO2>92%<95%.
Causes of study interruption were the following signs: poor tolerance, abdominal paradox, and f >30 breaths.min-1, severe desaturation (<90%) despite 8 l/min of oxygen or ab FiO2 greater than 0.4, heart rate >145 beats.min-1, or sustained increase or decrease in heart rate by more than 20%, arrhythmia, systolic blood pressure >180 or <70 mmHg at three consecutive measurements, agitation and anxiety, and diaphoresis.
Statistical analysis
Results are shown as mean±SD. Two-way analysis of variance was performed to assess the differences in physiological continuous variables between-condition (spontaneous breathing vs pressure support) and within-condition (T0, T1 and T2, and T030m, T130m, T230m) (2-way ANOVA for repeated measures). Friedman’s test was used to compare repeated measures of dyspnoea (VAS) in either condition. Paired t-test was employed to assess differences between means when appropriate. Computations were performed using SPSS 12.0 package (SPSS Chicago Ill., USA).
Results
During their stay in the WC ten out of 16 patients underwent decreasing levels of the pressure support protocol and six patients experienced increasing periods of spontaneous breathing. At the time of the study patients undergoing reduced levels of pressure support were assisted at a mean level of IPS=13±2 cmH20. Patients experiencing increasing periods of spontaneous breathing were spending 4±2 h/day in that condition. Table 1 shows anthropometrics and clinical characteristics of patients in the study. Four out of 16 patients were being treated with IV steroids, in eight patients the cause of admission in ICU was pneumonia, while in eight patients this was due to a relapse of their chronic disease. None of patients died in hospital before discharge. Eight patients were discharged from the WC with a tracheotomy and four of them had a home mechanical ventilation prescription. In the first day of the study, seven patients and nine patients were randomly assigned to assisted and unassisted ventilation, respectively. In the two days of study, meals consisted of similar caloric intake (542±130 kcal vs 590±140 kcal under spontaneous breathing and pressure support, respectively). The caloric intake during the studied meals represented 39±18% of the total scheduled calories according to REE. During the protocol (lasting 90 min), in six out of 16 patients no suction was performed on any study day; in eight out of 16 patients suction was performed both during inspiratory pressure support or during unassisted breathing, whereas two out of 16 patients needed to be suctioned under pressure support but not under spontaneous breathing. The mean number of suctions was similar: 0.8±1 vs 0.7±0.7 in spontaneous breathing and pressure support, respectively.
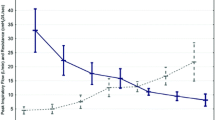
Figure 1 shows mean values of breathing pattern (respiratory rate, tidal volume, respiratory rate/tidal volume), end-tidal CO2, arterial saturation, and pulse rate either during pressure support or unassisted ventilation. Under unassisted breathing compared to baseline, ANOVA analysis showed a significant increase for respiratory rate (ANOVA time effects T= P<0.001) and for end-tidal CO2 (ANOVA time effects T= P<0.03) induced by the meal.
Mean values and SD of breathing pattern parameters, end-tidal CO2, O2 saturation, and heart rate before (T0), during (T1), and after meal-time (T2) under spontaneous breathing (SPONTANEOUS BREATHING= continuous line) and Inspiratory Pressure Support Ventilation (IPS = dashed line). All data are averages of 30-min continuous recording before (T0), during (T1), and after (T2) meal-time, respectively. (M) effect of modality of breathing in 2-way ANOVA for repeated measures; (T) effect of time in 2-way ANOVA for repeated measures.
Indeed, during the whole study session, assisted ventilation (ANOVA modality effect M) was associated with a significantly greater tidal volume (ANOVA M= P<0.001), lower respiratory rate (ANOVA M= P<0.032), lower respiratory rate/tidal volume (ANOVA M= P<0.001), and lower pulse rate (ANOVA M= P<0.047) than unassisted ventilation with a faster return to baseline condition. There was no mean change in arterial saturation observed under spontaneous breathing or pressure support. During unassisted breathing four patients showed rapid shallow breathing (respiratory rate/tidal volume >105) while eating. No patients showed this under pressure support.
Figure 2 shows individual (dashed lines) and mean values (continuous line) of VAS in patients under spontaneous breathing (left) and under assisted breathing (right) before, during, and after meals. During meals, patients under unassisted breathing showed a statistical worsening in dyspnoea sensation (Friedman’s ANOVA P<0.001) while this was not found under mechanical ventilation.
Individual data (dashed lines) and mean values (continuous lines) of dyspnoea sensation (as assessed by VAS) under unassisted (left panel) and assisted breathing (right panel) before, during, and after meal-time. A statistically significant worsening in VAS under unassisted (Friedman’s ANOVA P<0.001) but not under assisted breathing among the three observations was observed.
Discussion
This study is the first to show that in spontaneously breathing difficult-to-wean tracheostomised COPD patients: 1) under unassisted breathing, meals may induce increases in respiratory rate, end-tidal CO2, and dyspnoea; and 2) pressure support ventilation prevents dyspnoea from worsening during meals.
In the management of the ventilator-supported patient, nutritional support has been studied in terms of necessity of caloric intake, nitrogen balance, parenteral or enteral route, and related side effects. In severely difficult-to-wean COPD patients we [16] previously demonstrated that two different weaning protocols, namely, progressive reduction in inspiratory pressure support assistance or increasing periods of spontaneous breathing trials, resulted in similar weaning success rate and WC length of stay. Among the few activities of daily life these patients are able to perform during long periods of hospitalisation, meals often represent a critical moment for them as well as for nurses. Risk of dysphagia, arterial desaturation, respiratory distress, muscular fatigue, and inadequate dietary intake for energy expenditure may transform this moment into a highly stressful condition. Preliminary data [17] have suggested the occurrence of respiratory rate, heart rate, and dyspnoea variations during meal-time in tracheostomised patients breathing spontaneously. Nevertheless, there is a lack of information on physiological effects of meals in these patients not only in spontaneous breathing but also during assisted ventilation. At the same time, no study has been performed focussing on whether the meal-time may provoke or elicit subjective sensations of dyspnoea.
Smith et al. [1] studied the breathing pattern changes induced by eating and drinking in healthy adults. Although the level of ventilation remained constant during eating and drinking the pattern of breathing became increasingly irregular. This author concluded that an irregular pattern might contribute to dyspnoea during meals in some patients with lung disease [1]. Smith et al. [1] proposed that the increased work of breathing, combined with a reduction in Functional Residual Capacity, may in part be responsible for the dyspnoea on eating noted by some people with respiratory disease. In our study, patients also showed a more irregular-breathing pattern during meals as shown by a tendency to significantly change the breathing pattern (increase in respiratory rate and tidal volume), which did not change immediately after meals.
Patients with moderate-to-severe chronic disease such as post-acute stroke may present hypoxemia during eating [2]. Dysphagia is common after stroke and detection of silent aspirations is important because these events often lead to serious complications such as pulmonary infection, sepsis, dehydration, and malnutrition [2, 3, 4, 5, 6, 7]. It has been speculated that meal-related episodes of desaturation may occur as a result of aspiration of fluid/food into the airway, which may cause a mismatch of pulmonary ventilation/perfusion and, ultimately, hypoxemia [2, 4], and an incoordination of breathing [5, 6]. Physiological monitoring during eating might yield new insights on the pathophysiology of such serious complications in this particular clinical setting and the means to prevent them [7].
Some studies have described falls in SatO2 or in PaO2 during meals in patients with COPD [8, 9]. These patients with moderate-to-severe COPD may develop transient oxygen desaturation associated with modification of breathing during daily activities such as walking, washing, eating [11], defecation [10], and even during simple activities such as sweeping, erasing a blackboard, lifting pots, and replacing lamps [12] sometimes showing a latent chronic respiratory insufficiency.
Several mechanisms were proposed to explain desaturation during meals: patients showed a significant drop in saturation in the first minutes after the beginning of the meal and a significant increase immediately after the completion of the meal [14]. The fact that desaturation occurs so early after the beginning of the meal excludes possible metabolic effects of food absorption and digestion. In agreement with Schols et al. [14] 18% of our patients showed transient desaturations in the early phase of the meal with no cardiac effects as demonstrated by the stability in pulse rate. Otherwise, in the majority of studied patients, arterial desaturations were relatively small and of negligible haemodynamic consequence [11]. The present data have confirmed a lack of practical implications for meal-induced modifications on oxygenation probably due to the observed compensatory increase in respiratory rate and tidal volume.
The patients studied presented signs of hyperinflation demonstrated by a high residual volume before admission in ICU; in these patients the increase of end-expiratory lung volume is one of the most probable causes to explain the increase in breathing pattern and end-tidal CO2 during meals. On the other hand, it is usual to note a breathing pattern change in daily life activities. The respiratory centre controls breathing, which obviously varies, to regulate (to maintain constant) PaCO2 whenever is possible. Thus, the increase of minute ventilation with a meal may be considered as a good index (patients are probably able to regulate PaCO2 at the price of an increase in dyspnoea). In addition, it could be argued that patients overventilated by assisted mechanical ventilation in a basal condition, under the increase in ventilatory demand induced by the meal, were hidden by this assisted mechanical hyperventilation.
Weaning from artificial nutrition with subsequent possibility to spontaneously eat is an essential rehabilitative outcome in severely tracheostomised difficult-to-wean patients. This study indicates that the physiological consequences of meals may be not as severe as might be suspected by a worsening of dyspnoea. At the same time, this study suggests that physiological variations under meal are not dramatically different with or without mechanical ventilation.
The need of a better description of meal-times and other activities of daily life and the usefulness of monitoring subjective sensations of dyspnoea during weaning attempts have been emphasized by the present data. Further studies will focus on the short- and long-term consequences of the physiological and clinical changes found in this study.
Limitations of the study are the confounding effect of suction on the results and the lack of measurement of variations in end-expiratory lung volume.
In conclusion, in tracheostomised difficult-to-wean COPD patients: 1) under unassisted breathing, meals may induce increase in respiratory rate, end-tidal CO2 and dyspnoea; and 2) inspiratory pressure support ventilation prevents dyspnoea from worsening during meals.
References
Smith J, Wolkove N, Colacone A, Kreisman H (1989) Coordination of eating, drinking and breathing in adults. Chest 96:578–582
Roger B, Adverson J, Msall M, Suchard D (1993) Hypoxaemia during oral feeding in adults with dysphagia and severe neurological disabilities. Dysphagia 8:43–48
Roger B, Adverson J, Msall M, Demerath R (1993) Hypoxaemia during oral feeding in children with severe cerebral palsy. Dev Med Child Neurol 35:3–10
Zaidi NH, Smith HA, King SC, Park C, O’Neill PA, Connolly MJ (1995) Oxygen desaturation on swallowing as a potential marker of aspiration in acute stroke. Age Ageing 24:267–270
Good DC, Henkle JQ, Gelber D, Welsh J, Verulst S (1996) Sleep disordered breathing and poor functional outcome after stroke. Stroke 27:252–259
Teramoto S, Fukuky Y, Ouchi Y (1996) Oxygen desaturation on swallowing in patients with stroke: what does it mean? Age Ageing 25:333–334
Rowat AM, Wardlaw JM, Dennis MS, Warlow CP (2000) Does feeding after arterial oxygen saturation in patients with acute stroke? Stroke 31:2134–2140
Brown SE, Casciari RJ, Light RW (1983) Arterial oxygen saturation during meal in patients with severe chronic obstructive pulmonary disease. South Med J 76:194–198
Norregaard O, Larsen BO, Dahl R (1988) Meal-related changes in dyspnoea and arterial blood gases in patients suffering from COPD. Eur Respir J Dis 18 [Suppl 33]:68 s [abstr]
Soguel Schenkel N, Burdet L, de Muralt B, Fitting JW (1986) Oxygen saturation during daily activities in chronic obstructive pulmonary disease. Eur Respir J 9:2584–2589
Delmastro M, Santoro C, Nava S (2004) Respiratory changes during defecation in patients with chronic respiratory failure. Eur Respir J 23:617–619
Velloso M, Garcia Stella S, Cendon S, Carlos Silva A, Jardin JR (2003) Metabolic and ventilatory parameters of four activities of daily living accomplished with arms in COPD patients. Chest 123:1047–1053
Brown SR, Kmeim NL, Dixon RL, Clagnaz P, Anderegg A, Shrago ES (1984) The prevalence and determinants of nutritional changes in chronic obstructive pulmonary disease. Chest 86:558–563
Schols A, Monstert R, Cobben N, Soeters P, Wouters E (1991) Transcutaneous oxygen saturation and carbon dioxide tension during meals in patients with chronic obstructive pulmonary disease. Chest 95:1287–1292
Ambrosino N, Clini E (2004) Long-term mechanical ventilation and nutrition. Respir Med 98:413–420
Vitacca M, Vianello A, Colombo D, Clini E, Porta R, Bianchi L, Arcaro G, Guffanti E, Lo Coco A, Ambrosino N (2001) Comparison of two methods for weaning COPD patients requiring mechanical ventilation for more than 15 days. Am J Respir Crit Care Med 164:225–230
Barbano L, Callegari G, Porta R, Vitacca M, Ambrosino N (2001) Cardiorespiratory adaptations during meal-time in tracheostomized patients (TP) hospitalized for prolonged weaning. Eur Respir J 18 [Suppl 33]:389 s [abstr]
American Thoracic Society Statement (1995) Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 152:S77–S120
Sacnker MA, Watson H, Belsito AS, Feinerman D, Suarez M, Gonzalez G, Bizousky F, Krieger B (1989) Calibration of respiratory inductive plethysmograph during natural breathing. J Appl Physiol 66:410–420
Tobin MJ, Perez W, Guenther SM, Semmes BJ, Mador MJ, Allen SJ, Lodato RF, Dantzker DR (1986) The pattern of breathing during succesfull and unsuccesfull trials of weaning from mechanical ventilation. Am Rev Respir Dis 134:1111–1118
Aitken RC (1969) Measurement of feelings using Visual Analogue Scales. Proc R Soc Med 62:989–993
Liesker JJW, Wijkstra PJ, Ten Hacken NHT, Koeter GH, Postma DS, Kerstjens HAM (2002) A systemic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest 121:597–608
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vitacca, M., Callegari, G., Sarvà, M. et al. Physiological effects of meals in difficult-to-wean tracheostomised patients with chronic obstructive pulmonary disease. Intensive Care Med 31, 236–242 (2005). https://doi.org/10.1007/s00134-004-2530-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2530-z