Abstract
Background
This meta-analysis compared the effects of non-invasive ventilation (NIV) with invasive mechanical ventilation (InMV) and standard oxygen (O2) therapy on mortality and rate of tracheal intubation in patients presenting acute respiratory failure (ARF).
Methods
We searched the MEDLINE, EMBASE and Cochrane Central Register of clinical trials databases between 1949 and May 2015 to identify randomized trials of NIV for ARF. We excluded the ARF caused by extubation, cardiogenic pulmonary edema, and COPD.
Results
The meta-analysis included 21 studies and 1691 patients, of whom 846 were assigned to NIV and 845 to control (InMV or standard O2 therapy). One hundred ninety-one patients (22.6%) in the NIV group and 261 patients (30.9%) in the control group died before discharge from hospital. The pooled odds ratio (OR) for short-term mortality (in-hospital mortality) was 0.56 (95% CI 0.40–0.78). When comparing NIV with standard O2 therapy, the short-term mortality was 155 (27.4%) versus 204 (36.0%), respectively. For this comparison, the pooled OR of short-term mortality was 0.56 (95% CI 0.36–0.85). When comparing NIV with InMV, the short-term mortality was 36 (12.9%) versus 57 (20.5%) patients, respectively. For this comparison, the pooled OR of short-term mortality was 0.56 (95% CI 0.34–0.90). Tracheal intubation was performed in 106 patients (22.7%) in the NIV and in 183 patients (39.4%) in the standard O2 group, representing a pooled OR of 0.37 (95% CI 0.25–0.55). There were publication biases and the quality of the evidence was graded as low.
Conclusion
Compared with standard O2 therapy or InMV, NIV lowered both the short-term mortality and the rate of tracheal intubation in patients presenting with ARF.
Similar content being viewed by others
Introduction
Acute respiratory failure (ARF) is a widely prevalent medical emergency, which must be treated in a timely manner to prevent developments of life-threatening hypoxia or/and hypercapnia [1]. Non-invasive ventilation (NIV) is one of the treatments of hypoxic respiratory failure, which effectively improves the gas exchange in selected patients although it is still unknown if it affects the prognosis [2].
The first report of NIV, used in 10 patients presenting with ARF due to intrinsic diseases, was published in 1989 [3]. Since then, NIV has been widely used because it did not require tracheal intubations and/or specialized medical personnel, which seemed to save time and lower costs. The mask was generally well tolerated and there were no obvious complications, such as vomiting or aspiration. In addition, the physiologic improvements observed in that study were similar to those achieved with intubation and mechanical ventilation. After the benefits conferred by NIV observed in that study, several randomized controlled trials (RCTs) confirmed that NIV prevented the need for tracheal intubation and increased the blood oxygen (O2) concentration in hypoxic patients [4,5,6]. However, the effects of NIV on mortality rate have remained unknown. Some have claimed that it increases the survival rate in the acute care setting, while others have stated that it does not increase survival since failure of NIV management is associated with a significantly higher mortality [7,8,9,10,11,12]. The controversial results of the previous studies regarding the potential benefits of NIV may be explained by the variable degree of hypoxia. Systematic reviews to assess the efficacy of NIV for chronic obstructive pulmonary disease (COPD) or cardiogenic pulmonary edema (CPE) demonstrated its efficacy and safety [13, 14]. However, it has remained unknown whether NIV is effective among patients with acute respiratory failure, excluding ARF due to these etiologies.
This study examined whether NIV increases the survival rate of patients presenting with ARF excluding COPD and CPE.
The efficacy of NIV is mainly attributed to increasing lung volumes and decreasing work of breathing of the patients [15]. It is, therefore, currently considered first-line treatment of disorders such as post-extubation respiratory failure (PERF), CPE, and exacerbation of COPD, in which several prospective studies have confirmed its efficacy [16,17,18]. NIV lowered the risk of intubation by 65%, and the length of hospitalization by 1.9 days compared with InMV [16]. However, these distinct disorders, particularly CPE, are triggered by cardiac diseases rather than by respiratory failure. PERF and COPD are not limited to lung tissue. Therefore, after excluding these 3 etiologies of ARF, we want to discuss the majority of patients who presented with “pure” ARF: this is an important difference compared to other previous systematic reviews [9, 10].
NIV is contraindicated in patients presenting with respiratory arrest or upper airway obstructions when: the mask does not fit or the secretions cannot be managed properly; compliance with the mask is poor; or there is instability in hemodynamic status. Several complications associated with failure of treatment have also been reported, such as skin lesions or major air leaks [7, 19]. The failure of NIV may influence the intubation rate and mortality, and is more prevalent in ARF complicated by hypoxia [20]. The NIV failure rate has ranged from 10 to 40% among various studies [21], suggesting that its outcome is influenced by personal experiences or techniques. Its effectiveness and the benefit it has conferred on survival has, indeed, been variable among medical centers and experimental protocols designed to standardize its use and mitigate the variability of judgment and decision-making among caregivers [22]. Consequently, a meta-analysis of randomized trials seemed the best means of resolving differences attributable to the temporal variability in the collection of data by different institutions and countries. The aim of this meta-analysis was to examine the effects of NIV on mortality and tracheal intubation rate in ARF not due to PERF, CPE or exacerbation of COPD, with a view to help caregivers choose an optimal first-line of treatment in specific clinical settings.
Methods
Data sources and search strategies
We searched the MEDLINE, EMBASE and the Cochrane Central Register databases of clinical trials, published between January 1949 and 6 May 2015, January 1949 and 2 June 2015, and January 1949 and 1 June 2015, respectively. The full search strategies for each database are described in the Online Supplement 1.
Study selection
The titles and abstracts of references retrieved from the databases, and literature searches were independently conducted by the four investigators (YK, JK, AK, RS), prior to the full article reviews. Divergences of opinion were resolved by consensus. We used the following criteria to identify studies to be included: (1) randomized trial design; (2) inclusion of patients presenting with ARF and hypoxemia, defined by each study; (3) comparisons of NIV with mechanical ventilation or standard O2 therapy for treatment of ARF. The exclusion criteria for studies were: (1) < 18 years of age; (2) patients with CPE as a single etiology; (3) patients with exacerbation of COPD as a single etiology; (4) ARF following extubation; (5) studies performed in an ambulatory setting; (6) studies published in a language other than English.
Data extraction
The four investigators independently extracted the data from each eligible study. We contacted the corresponding authors of eligible articles via e-mail to request missing data. The data extracted included: author, year of publication, study design, number of patients, interventions (NIV, invasive mechanical ventilation and standard O2 therapy), outcome measures and study results, including (a) short-term mortality, defined as death in the intensive care unit or in the hospital, and (b) tracheal intubation. Divergences of opinion were resolved by consensus.
Study endpoints
The primary outcome was short-term mortality as defined earlier in each of the following comparisons: (1) NIV versus InMV or standard O2 therapy, (2) NIV versus standard O2 therapy defined as any oxygen concentration delivered by mask, (3) NIV versus InMV. The secondary outcome was tracheal intubation rate in the non-invasive versus standard O2 therapy comparison.
Assessment of methodological quality: risk of bias assessment and GRADE approach
We adapted the Cochrane risk of bias tool to assess the quality of the studies included in the meta-analysis [23, 24]. Each study was assessed for: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and staff (performance bias); (4) blinding of related outcomes assessment (detection bias); (5) incomplete outcome data (attrition bias); (6) selective reporting (reporting bias); (7) other biases. We classified the studies as low, intermediate or high risk of bias in each domain. In addition, we graded the quality of evidence of each finding based on the criteria established by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group [25]. The quality of the study methodology was classified by the four independent investigators as high, intermediate, low or very low, based on the study design, risk of biases, indirectness, inconsistency, imprecision and publication bias. The publication biases were assessed visually by inspecting funnel plots as well as analytical appraisals based on Eggar’s linear regression test [26]. A two-sided p value ≤ 0.10 was regarded as significant in Eggar’s linear regression test.
Statistical analysis
We pooled the eligible patients for each outcome and calculated the odds ratios (OR) and corresponding 95% confidence intervals (CI), using the Der Simonian–Laird random-effects model with weights calculated by the inverse variance method. We verified the heterogeneity of the studies, using the estimated Cochrane chi-square test and the I 2 statistic with I 2 > 50%.
We applied unadjusted p values for the significance assessment in this study. It was set at the two-tailed 0.05 level for hypothesis testing and at the 0.10 level for testing of heterogeneity.
The meta-analyses were performed using the Review Manager, Cochrane systematic review software, version 5.3.5 for Windows (Copenhagen: The Nordic Cochrane Centre; The Cochrane Collaboration, 2014, http://tech.cochrane.org/revman). The publication biases were analyzed with Stata version 13® (Stata Corp LP, 2013).
Results
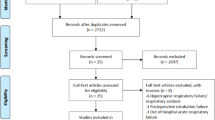
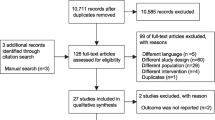
We identified 2482 studies from the electronic databases after elimination of duplicates. We excluded 1626 studies because their design was not randomized, 546 studies because the patients did not fit our selection criteria, and 259 studies because they were not published in English. We retained 51 studies for review of the full-length reports, and included 21 studies [5, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] in the final analysis (Fig. 1).
Study characteristics
The 21 trials [5, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] included 1691 patients, of whom 846 were randomly assigned to NIV and 845 to controls. In 17 trials [5, 28,29,30,31,32,33, 35,36,37, 39, 41,42,43,44,45,46] 566 patients were assigned to NIV and 567 were assigned to standard O2 therapy. In four trials [27, 34, 38, 40] 280 patients were assigned to NIV and 278 to invasive ventilation. In all trials, patients were assigned each intervention as an initial therapy for respiratory failure, and patients who received NIV or InMV before assignment were excluded from each study. Among the 17 trials, which compared NIV with standard O2 therapy, two [31, 35] reported no death. Of these 17 trials, 16 [5, 28,29,30,31,32,33, 35,36,37, 39, 42,43,44,45,46] reported tracheal intubation as a secondary outcome and two [31, 35] of these 16 trials reported no instance of intubation. The non-invasive ventilators used were BiPAP Vision® (Respironics Inc. Koninklijke Philips N.V., Amsterdam, The Netherlands) in 6 studies (28.6%), Puritan Bennett™ 7200® (Covidian, Minneapolis, MN) in 5 (23.8%), and Dräger Evita® Infinity® V500 (Drägerwerk AG & Co. KGaA, Lübeck, Germany) in 4 studies (19.0%). The NIV interfaces were full-face masks in 11 studies (52.4%), nasal masks in 5 (23.8%), face masks in 4 (19.1%), helmets in 3 (14.3%), and an oro-nasal mask in 1 study (4.8%). The NIV mode was BiPAP in 15 (71.4%) and CPAP in 6 studies (28.6%). The individual characteristics of the trials included in this meta-analysis are detailed in Table 1.
Outcomes
In the NIV group, 191 patients (22.6%) died in the hospital, before or after leaving the intensive care unit, while 261 patients (30.9%) died in the hospital during standard O2 therapy or InMV. The pooled OR of short-term mortality (Fig. 2) was 0.56 (95% CI 0.40–0.78). When comparing NIV with standard O2 therapy, the short-term mortality was 155 patients (27.4%) versus 204 (36.0%) patients, respectively. For this comparison (Fig. 3), the pooled OR of short-term mortality was 0.56 (95% CI 0.36–0.85). When comparing NIV with InMV, the short-term mortality was 36 patients (12.9%) versus 57 (20.5%) patients, respectively. For this comparison (Fig. 4), the pooled OR of short-term mortality was 0.56 (95% CI 0.34–0.90). Tracheal intubation (Fig. 5) was performed in 106 patients (22.7%) in the NIV and in 183 patients (39.4%) in the standard O2 group, representing a pooled OR of 0.37 (95% CI 0.25–0.55).
Heterogeneity
A statistically significant heterogeneity in short-term mortality was observed between the NIV and the control (standard O2 therapy and InMV) groups (I 2 = 34.0%, χ 2 = 27.3, p = 0.07) and between the NIV and the standard O2 groups (I 2 = 42.0%, χ 2 = 24.3, p = 0.04). A statistical heterogeneity was observed in neither short-term mortality between the NIV and the InMV groups (I 2 = 0.0%; χ 2 = 1.95; p = 0.58) nor in the tracheal intubation rate between the NIV and the standard O2 groups (I 2 = 28.0%, χ 2 = 19.3, p = 0.15).
Publication biases, risk of bias and quality of evidence
We tested for the presence of publication biases for each outcome, except the short-term mortality between the NIV and the invasive ventilation group, because of a small sample size. A visual inspection of the funnel plots and the Eggar linear regression test suggested the existence of publication biases in short-term mortality (Figs. 6, 7) between (a) the NIV and the control (standard O2 therapy and InMV) groups (p < 0.01) and (b) the NIV and standard O2 therapy group (p < 0.01). The funnel plot for the tracheal intubation rate between the NIV and standard O2 groups was symmetric (Fig. 8), excluding the existence of a small publication bias (p = 0.10).
In the nature of the intervention, blinding was categorized as high risk for all the trials and selective outcome reporting was assessed as uncertain risk for nearly all the trials due to the unavailability of study protocols (Online Supplements 2, 3 and 4). The quality of evidence was rated as low for the effect of NIV on short-term mortality compared with the control group, including standard O2 therapy or InMV. The grade was lowered by 2 points, due to the major inconsistency and publication bias, for which the Cochrane chi-square test showed a significant heterogeneity. The quality of evidence was rated as low for the effect of NIV on short-term mortality, compared with standard O2 therapy. The grade was lowered by 2 points due to a very serious risk of bias; the domain of blinding in risk of bias was rated as high in nearly all studies because the decision of intubation depended on each clinician. The quality of evidence was rated as low for the effect of NIV on short-term mortality compared with invasive ventilation. We lowered the grade by 2 points because of a major imprecision, since the size of the criterion of optimal information was insufficient, and because of a publication bias (Table 2). The detail of the evidence profile is shown in Online Supplement 5.
Discussion
This meta-analysis of 21 randomized trials, which includes 1691 patients with ARF, suggests that NIV lowers the short-term mortality compared with standard O2 therapy and InMV. Subgroup analyses were performed comparing NIV with the standard O2 therapy and InMV control groups separately. The short-term mortality was significantly higher in both control groups than in the NIV group. In addition, NIV lowered the tracheal intubation rate. We excluded patients presenting with PERF, CPE or exacerbation of COPD to discuss only ARF caused by lung disease such as ARDS.
Previous meta-analyses [9,10,11,12,13,14, 16, 39] of patients presenting with ARF have been published, which have reported favorable survival rates attributable mainly to the inclusion of PERF, CPE and exacerbation of COPD [22, 47, 48]. On the other hand, this is the first study which evaluates the efficacy of NIV among patients with ARF excluding CPE or COPD. Because of the paucity of randomized trials limited to acute lung injury ALI/ARDS, we are aware of only two relevant meta-analyses of NIV for this indication. In 2010, Agarwal et al. examined the role of NIV in the management of ALI and ARDS [49]. They searched the Pubmed and EMBASE databases for relevant studies published between 1995 and 2009, and included studies that reported rates of tracheal intubation, or death or both in patients with ALI/ARDS treated with NIV. They found 13 studies, including a total of 540 patients who met their inclusion criteria. Their analysis revealed a nearly 50% failure of NIV, prompting the authors to conclude that NIV should be used cautiously in this population. They also found a significant statistical heterogeneity for both intubations (I 2 = 76%, 95% CI 55–85, Cochran Q statistic 50, p = 0.001) and deaths (I 2 = 79%, 95% CI 61–86, Cochran Q statistic 56, p = 0.001). The quality of that analysis was lowered by the inclusion of several observational studies and by the presentation of insufficient overall evidence [49]. In 2014, Luo et al. pointed out in their meta-analysis that the role of NIV in the management of ALI/ARDS was controversial [50]. They included studies that reported the tracheal intubation rate and/or mortality in patients treated for ALI/ARDS with NIV. They found 6 RCTs, including a total of 227 patients who met their inclusion criteria. In their meta-analysis, they did not find an improvement of in-hospital mortality, although the rate of tracheal intubation was significantly lowered by NIV. The heterogeneity for tracheal intubation and in-hospital mortality was (I 2 = 43%, χ 2 = 8.82, p = 0.12) and (I 2 = 61%, χ 2 = 5.12, p = 0.08), respectively [50]. These two studies were both limited by the small sample sizes and by the inclusion of high heterogeneity only, which may explain the absence of effects on the survival rate. To obviate these limitations, we included 21 studies dedicated to the treatments, including nearly 1700 patients with ARF, and excluded patients suffering from PERF, CPE or COPD. This large sample size, combined with the lower heterogeneity rates observed in our study (compared with the studies of Agarwal et al. and Luo et al.) increased the reliability of the outcome estimate, in which we found significantly lower rates of both short-term mortality and tracheal intubation conferred by NIV.
Another contribution of our study was in the comparisons between NIV with InMV. Several studies have been limited to comparisons of NIV with standard O2 therapy, although it is important to compare non-invasive versus InMV in clinical settings such as emergency departments or intensive care units [49,50,51]. It has been suggested that the failure of NIV in patients with ARF is independently correlated with poor outcomes compared with patients intubated without prior NIV [20]. In our study, short-term mortality was significantly lower in the NIV than in the InMV group.
From a physiological perspective, the benefits conferred by NIV are not only from continuous positive end-expiratory pressure, since both NIV and InMV can create physiological conditions of positive end-expiratory pressure. NIV interferes with neither the native upper airways nor the glottis function. It can alleviate the respiratory efforts and improve gas exchange while preserving the ability to swallow, cough, and speak. Furthermore, NIV may obviate the adverse effects of InMV, including deep sedation, administration of muscle relaxants, delirium, ventilator-induced lung injury, and ventilator-associated infections [52, 53].
Limitations of our study
All randomized trials included in our meta-analysis were not double-blinded because the NIV is a visible intervention and cannot be concealed. Therefore, we assigned a high risk of bias to all the trials. Furthermore, the publication bias and selective outcome reporting were assessed as unclear risks of bias because we could not obtain the actual study protocols for most of the studies. A second limitation of this meta-analysis was the inclusion of various kinds of diseases causing ARF, as well as obvious differences in the patients’ baseline characteristics among the studies. Third, treatment and prognosis of ARF are changing year by year and it is no longer the same situation as in the old days. Finally, our analysis was limited to short-term mortality because most studies did not report long-term survival data. Whether a decrease in short-term mortality increases the long-term survival remains to be clarified by further investigations.
Conclusion
In this meta-analysis of 21 randomized trials and 1691 patients with ARF, NIV lowered both the short-term mortality and the rate of tracheal intubation compared with standard O2 or InMV. Despite unavoidable risks of biases, this study suggests that NIV is worth considering as a treatment option for patients with ARF.
Abbreviations
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- ARF:
-
Acute respiratory failure
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- CPAP:
-
Continuous positive airway pressure
- CPE:
-
Cardiogenic pulmonary oedema
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- NIV:
-
Non-invasive ventilation
- OR:
-
Odds ratio
- PEEP:
-
Positive end expiratory pressure
- PERF:
-
Post-extubation respiratory failure
References
Azoulay E, Thiery G, Chevret S, Moreau D, Darmon M, Bergeron A, Yang K, Meignin V, Ciroldi M, Le Gall JR, Tazi A, Schlemmer B. The prognosis of acute respiratory failure in critically ill cancer patients. Medicine. 2004;83:360–70.
Yu KY, Zhao L, Chen Z, Yang M. Noninvasive positive pressure ventilation for the treatment of acute respiratory distress syndrome following esophagectomy for esophageal cancer: a clinical comparative study. J Thorac Dis. 2013;5:777–82.
Meduri GU, Conoscenti CC, Menashe P, Nair S. Noninvasive face mask ventilation in patients with acute respiratory failure. Chest. 1989;95:865–70.
Kohnlein T, Windisch W, Kohler D, Drabik A, Geiseler J, Hartl S, Karg O, Laier-Groeneveld G, Nava S, Schonhofer B, Schucher B, Wegscheider K, Criee CP, Welte T. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2:698–705.
Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995;151:1799–806.
Masip J, Betbese AJ, Paez J, Vecilla F, Canizares R, Padro J, Paz MA, de Otero J, Ballus J. Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trial. Lancet (London, England). 2000;356:2126–32.
Cabrini L, Nobile L, Plumari VP, Landoni G, Borghi G, Mucchetti M, Zangrillo A. Intraoperative prophylactic and therapeutic non-invasive ventilation: a systematic review. Br J Anaesth. 2014;112:638–47.
Demoule A, Girou E, Richard JC, Taille S, Brochard L. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med. 2006;32:1756–65.
Liu YJ, Zhao J, Tang H. Non-invasive ventilation in acute respiratory failure: a meta-analysis. Clin Med (London, England). 2016;16:514–23.
Wang T, Zhang L, Luo K, He J, Ma Y, Li Z, Zhao N, Xu Q, Li Y, Yu X. Noninvasive versus invasive mechanical ventilation for immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. BMC Pulm Med. 2016;16:129.
Liu Q, Gao Y, Chen R, Cheng Z. Noninvasive ventilation with helmet versus control strategy in patients with acute respiratory failure: a systematic review and meta-analysis of controlled studies. Crit Care (London, England). 2016;20:265.
Amado-Rodriguez L, Bernal T, Lopez-Alonso I, Blazquez-Prieto J, Garcia-Prieto E, Albaiceta GM. Impact of initial ventilatory strategy in hematological patients with acute respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2016;44:1406–13.
Ram FS, Picot J, Lightowler J and Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(3):CD004104.
Vital FM, Ladeira MT and Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;(5):CD005351.
Berg KM, Lang GR, Salciccioli JD, Bak E, Cocchi MN, Gautam S, Donnino MW. The rapid shallow breathing index as a predictor of failure of noninvasive ventilation for patients with acute respiratory failure. Respir Care. 2012;57:1548–54.
Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and meta-analysis. Chest. 2008;133:756–66.
Kilger E, Briegel J, Haller M, Frey L, Schelling G, Stoll C, Pichler B, Peter K. Effects of noninvasive positive pressure ventilatory support in non-COPD patients with acute respiratory insufficiency after early extubation. Intensive Care Med. 1999;25:1374–80.
Hoffmann B, Jepsen M, Hachenberg T, Huth C, Welte T. Cardiopulmonary effects of non-invasive positive pressure ventilation (NPPV)—a controlled, prospective study. Thorac Cardiovasc Surg. 2003;51:142–6.
Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet (London, England). 2009;374:250–9.
Thille AW, Contou D, Fragnoli C, Cordoba-Izquierdo A, Boissier F, Brun-Buisson C. Non-invasive ventilation for acute hypoxemic respiratory failure: intubation rate and risk factors. Crit Care (London, England). 2013;17:R269.
Charlesworth M, Elliott MW, Holmes JD. Noninvasive positive pressure ventilation for acute respiratory failure in delirious patients: understudied, underreported, or underappreciated? A systematic review and meta-analysis. Lung. 2012;190:597–603.
Cabrini L, Landoni G, Oriani A, Plumari VP, Nobile L, Greco M, Pasin L, Beretta L, Zangrillo A. Noninvasive ventilation and survival in acute care settings: a comprehensive systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2015;43:880–8.
Higgins JP, Altman DG on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions 2011. 2016. http://www.cochrane-handbook.org. Accessed 19 July 2017
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2011;343:d5928.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, deBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schunemann HJ. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315:629–34.
Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, Gasparetto A, Meduri GU. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339:429–35.
Antonelli M, Conti G, Bufi M, Costa MG, Lappa A, Rocco M, Gasparetto A, Meduri GU. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000;283:235–41.
Brambilla AM, Aliberti S, Prina E, Nicoli F, Del Forno M, Nava S, Ferrari G, Corradi F, Pelosi P, Bignamini A, Tarsia P, Cosentini R. Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med. 2014;40:942–9.
Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto Meduri G. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160:1585–91.
Cosentini R, Brambilla AM, Aliberti S, Bignamini A, Nava S, Maffei A, Martinotti R, Tarsia P, Monzani V, Pelosi P. Helmet continuous positive airway pressure vs oxygen therapy to improve oxygenation in community-acquired pneumonia: a randomized, controlled trial. Chest. 2010;138:114–20.
Delclaux C, L’Her E, Alberti C, Mancebo J, Abroug F, Conti G, Guérin C, Schortgen F, Lefort Y, Antonelli M, Lepage E, Lemaire F, Brochard L. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284:2352–60.
Ferrer M, Esquinas A, Leon M, Gonzalez G, Alarcon A, Torres A. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med. 2003;168:1438–44.
Gunduz M, Unlugenc H, Ozalevli M, Inanoglu K, Akman H. A comparative study of continuous positive airway pressure (CPAP) and intermittent positive pressure ventilation (IPPV) in patients with flail chest. Emerg Med J. 2005;22:325–9.
Gupta D, Nath A, Agarwal R, Behera D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respir Care. 2010;55:536–43.
Hernandez G, Fernandez R, Lopez-Reina P, Cuena R, Pedrosa A, Ortiz R, Hiradier P. Noninvasive ventilation reduces intubation in chest trauma-related hypoxemia: a randomized clinical trial. Chest. 2010;137:74–80.
Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, Reiffers J, Cardinaud JP. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–7.
Honrubia T, García López FJ, Franco N, Mas M, Guevara M, Daguerre M, Alía I, Algora A, Galdos P. Noninvasive vs conventional mechanical ventilation in acute respiratory failure: a multicenter, randomized controlled trial. Chest. 2005;128:3916–24.
Martin TJ, Hovis JD, Costantino JP, Bierman MI, Donahoe MP, Rogers RM, Kreit JW, Sciurba FC, Stiller RA, Sanders MH. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. Am J Respir Crit Care Med. 2000;161:807–13.
Matić I, Pavicić F, Sakić-Zdravcević K, Danić D, Jurjević M. Pulmonary compliance values provide prognosis in mechanically ventilated patients—a randomized prospective study. Coll Antropol. 2007;31:829–36.
Nava S, Ferrer M, Esquinas A, Scala R, Groff P, Cosentini R, Guido D, Lin CH, Cuomo AM, Grassi M. Palliative use of non-invasive ventilation in end-of-life patients with solid tumours: a randomised feasibility trial. Lancet Oncol. 2013;14:219–27.
Squadrone V, Massaia M, Bruno B, Marmont F, Falda M, Bagna C, Bertone S, Filippini C, Slutsky AS, Vitolo U, Boccadoro M, Ranieri VM. Early CPAP prevents evolution of acute lung injury in patients with hematologic malignancy. Intensive Care Med. 2010;36:1666–74.
Wermke M, Schiemanck S, Höffken G, Ehninger G, Bornhäuser M, Illmer T. Respiratory failure in patients undergoing allogeneic hematopoietic SCT—a randomized trial on early non-invasive ventilation based on standard care hematology wards. Bone Marrow Transplant. 2012;47:574–80.
Wood KA, Lewis L, Von Harz B, Kollef MH. The use of noninvasive positive pressure ventilation in the emergency department: results of a randomized clinical trial. Chest. 1998;113:1339–46.
Wysocki M, Tric L, Wolff MA, Millet H, Herman B. Noninvasive pressure support ventilation in patients with acute respiratory failure. A randomized comparison with conventional therapy. Chest. 1995;107:761–8.
Zhan Q, Sun B, Liang L, Yan X, Zhang L, Yang J, Wang L, Ma Z, Shi L, Wei L, Li G, Yang L, Shi Z, Chen Y, Xu Q, Li W, Zhu X, Wang Z, Sun Y, Zhuo J, Liu Y, Li X, Wang C. Early use of noninvasive positive pressure ventilation for acute lung injury: a multicenter randomized controlled trial. Crit Care Med. 2012;40:455–60.
Keenan SP, Sinuff T, Burns KE, Muscedere J, Kutsogiannis J, Mehta S, Cook DJ, Ayas N, Adhikari NK, Hand L, Scales DC, Pagnotta R, Lazosky L, Rocker G, Dial S, Laupland K, Sanders K, Dodek P. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ Can Med Assoc J (journal de l’Association medicale canadienne). 2011;183:E195–214.
Thille AW, Boissier F, Ben-Ghezala H, Razazi K, Mekontso-Dessap A, Brun-Buisson C, Brochard L. Easily identified at-risk patients for extubation failure may benefit from noninvasive ventilation: a prospective before-after study. Crit Care (London, England). 2016;20:48.
Agarwal R, Aggarwal AN, Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: a proportion meta-analysis. Respir Care. 2010;55:1653–60.
Luo J, Wang MY, Zhu H, Liang BM, Liu D, Peng XY, Wang RC, Li CT, He CY, Liang ZA. Can non-invasive positive pressure ventilation prevent endotracheal intubation in acute lung injury/acute respiratory distress syndrome? A meta-analysis. Respirology (Carlton, Vic). 2014;19:1149–57.
Lin C, Yu H, Fan H, Li Z. The efficacy of noninvasive ventilation in managing postextubation respiratory failure: a meta-analysis. Heart Lung J Crit Care. 2014;43:99–104.
Gregoretti C, Pisani L, Cortegiani A, Ranieri VM. Noninvasive ventilation in critically ill patients. Crit Care Clin. 2015;31:435–57.
Bouadma L, Sonneville R, Garrouste-Orgeas M, Darmon M, Souweine B, Voiriot G, Kallel H, Schwebel C, Goldgran-Toledano D, Dumenil AS, Argaud L, Ruckly S, Jamali S, Planquette B, Adrie C, Lucet JC, Azoulay E, Timsit JF. Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit Care Med. 2015;43:1798–806.
Acknowledgements
We thank all members of the Clinical Practice Guideline for ARDS 2016, from the Japanese Society of Respiratory Care Medicine, the Japanese Society of Intensive Care Medicine, and the Japanese Respiratory Society, as well as the librarians Yumi Yamashita, Yoshiko Nakagawa and Takaaki Suzuki, from Kyoto Prefectural University of Medicine library (YY and YN) and Nara Medical University library (TS), for their assistance in the search for relevant publications. Rodolphe Ruffy, M.D., http://www.cardioscript.com, reviewed the manuscript for style and language and was compensated for his services.
Author information
Authors and Affiliations
Contributions
YK, JK, AK, and RS designed the study. YK, JK, AK, and RS identified the studies entered in the meta-analysis, and extracted and analysed the data. YK and JK drafted the manuscript. AK, RS, EN and SH critically reviewed the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data and material used for this meta-analysis are contained in references [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] of our list of references.
Conflict of interest
The authors have no competing interest to declare.
Funding
This study was supported in part the Grant-in-Aid for Scientific Research (B) 24390404, 2012–2016, awarded to Satoru Hashimoto, M.D., Ph.D., by the Japanese Ministry of Education, Science, Sports and Culture.
Electronic supplementary material
Below are the links to the electronic supplementary material.
About this article
Cite this article
Kondo, Y., Kumasawa, J., Kawaguchi, A. et al. Effects of non-invasive ventilation in patients with acute respiratory failure excluding post-extubation respiratory failure, cardiogenic pulmonary edema and exacerbation of COPD: a systematic review and meta-analysis. J Anesth 31, 714–725 (2017). https://doi.org/10.1007/s00540-017-2389-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-017-2389-0