Article Text
Abstract
Background The associations between exposure to air pollution and asthma control are not well known. The objective of this study was to assess the association between long-term exposure to NO2, O3 and PM10 and asthma control in the follow-up of the Epidemiological study on the Genetics and Environment of Asthma (EGEA2) (2003–2007).
Methods Modelled outdoor NO2, O3 and PM10 estimates were linked to each residential address using the 4 km grid air pollutant surface developed by the French Institute of Environment in 2004. Asthma control was assessed in 481 subjects with current asthma using a multidimensional approach following the 2006–2009 Global Initiative for Asthma guidelines. Multinomial and ordinal logistic regressions were conducted adjusted for sex, age, body mass index, education, smoking and use of inhaled corticosteroids. The association between air pollution and the three domains of asthma control (symptoms, exacerbations and lung function) was assessed. ORs are reported per IQR.
Results Median concentrations (in micrograms per cubic metre) were 32 (IQR 25–38) for NO2 (n=465), 46 (41–52) for O3 and 21 (18–21) for PM10 (n=481). In total, 44%, 29% and 27% had controlled, partly controlled and uncontrolled asthma, respectively. The ordinal ORs for O3 and PM10 with asthma control were 1.69 (95% CI 1.22 to 2.34) and 1.35 (95% CI 1.13 to 1.64), respectively. When including both pollutants in the same model, both associations persisted. Associations were not modified by sex, smoking status, use of inhaled corticosteroids, atopy, season of examination or body mass index. Both pollutants were associated with each of the three main domains of control.
Conclusions The results suggest that long-term exposure to PM10 and O3 is associated with uncontrolled asthma in adults, defined by symptoms, exacerbations and lung function.
- Air pollution
- asthma
- asthma control
Statistics from Altmetric.com
Introduction
Asthma control reflects disease activity over a short period of time. Achieving and maintaining the control of asthma is a major goal in asthma management.1 ,2 Acute exposure to air pollution is associated with adverse respiratory effects in asthmatic adults, including increased respiratory symptoms or hospitalisations, or reduced lung function.3–5 Evidence showing the effects of chronic exposure to air pollution on asthma incidence in adults is growing.6–8 However, studies on the effect of chronic exposure to air pollution on asthma control are scant. In two studies, air pollution exposure was associated with the occurrence of asthma-like symptoms in the last 3 months and with emergency department visits and/or hospitalisations in the last year.9 ,10 In the first survey of the French Epidemiological study on the Genetics and Environment of Asthma (EGEA), higher exposure to O3 was associated with more severe asthma in adults.11
Following clinical guidelines, asthma control integrates several dimensions, reflecting acute and chronic activity of the disease: daytime and night-time symptoms, the need for rescue treatment, exacerbations in the past year and lung function.1 ,2 Although worldwide about half of the patients have poorly controlled disease,12–14 few epidemiological studies have been conducted to identify the determinants of asthma control, and even fewer have taken into account such a comprehensive measure of asthma control.12 ,15
From the data in EGEA2 (follow-up of EGEA), we propose to study the effect of long-term exposure to air pollution, using yearly modelled estimates, on asthma control, assessed by single criteria and by combining several domains of control to follow as closely as possible the Global Initiative for Asthma (GINA) 2006–2009.15
Methods
Study design
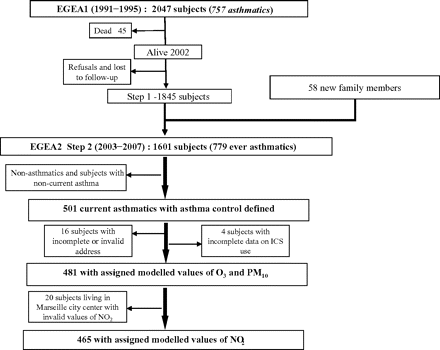
EGEA is an asthma case–control and family study (including spouses and first-degree relatives of the cases), conducted for the first time between 1991 and 1995 on 2047 subjects from five French cities. This analysis is based on the cross-sectional data of the follow-up study (EGEA2) conducted between 2003 and 2007 on 1571 adults.15–17 (figure 1, online supplement).
Flow chart of the Epidemiological study on the Genetics and Environment of Asthma (EGEA) population included in this analysis.
Asthma control
We defined asthma control in this population as previously published15 following GINA 2006–2009 guidelines.1 ,2 Asthma control was studied in subjects with current asthma, as done in previous publications,6 ,15 based on self-reports of respiratory symptoms over the past 3 months (ie, any of wheezing, nocturnal chest tightness, attack of shortness of breath following strenuous activity, at rest or at night, asthma attacks), use of short-acting β2 agonist inhalers in the past 3 months, exacerbation (oral steroid use or hospitalisation) in the past 12 months and FEV1. Subjects with controlled asthma were those without any of the following features: diurnal symptoms ≥1/week in the past 3 months, asthma attack in the past 3 months, nocturnal symptoms (woken due to asthma or by an attack of shortness of breath) in the past 3 months, use of short-acting β2 agonist inhalers ≥2/week in the past 3 months, use of oral corticosteroids in the past year and FEV1 <80% predicted. Subjects with partly controlled asthma were those with one or two of the above-mentioned features. Subjects with uncontrolled asthma were those with three or more of the above-mentioned features or those for whom respiratory problems had caused hospital or emergency admissions in the past year or had used oral corticosteroids in the past year or had 12 asthma attacks in the past 3 months.
Air pollution
Yearly estimates of outdoor air pollution concentrations at each participant's home address in 2004 for O3, PM10 (available for 481 of the 501 subjects with current asthma) and NO2 (available for 465 subjects) were derived from a geostatistical model by the French Institute of Environment. Interpolation was done for annual mean concentrations coming from background monitoring stations on a 4 km×4 km grid covering France. The residential addresses of all participants were geocoded to assign estimated annual means for O3-an, NO2 and PM10 as well as summer ozone (O3-sum) assessed from the monthly means from April to September. This has been previously described11 ,18 (online supplement).
Statistical analysis
First, the association between asthma control and air pollutants was expressed by ORs (reported for one IQR of the pollutant), derived from multinomial logistic regression. For each factor, the simultaneous assessment of the risk for uncontrolled asthma and for partly controlled asthma was compared with controlled asthma. Concerning the pollutants for which the proportional odds assumption hypothesis was verified in our data (O3-sum and PM10), we used the ordinal logistic regression leading to the estimate of a single OR that gives the risk to go from controlled to partly controlled asthma and from partly controlled to uncontrolled asthma. Generalised linear mixed models were also conducted in order to take into account the family structure and the city dependence of the population. The model integrates that there is a correlation between the subjects living in the same family (ie, all members of the family sharing genetic background and/or lifestyle determinants) or between the subjects living in the same city (ie, all participants living in the same city being exposed to the same background concentrations of air pollution). The effect of the family structure or city dependence is random, while the other effects of the model are fixed.
The crude associations are presented first, followed by the multivariate analysis, for each pollutant separately. Variables taken into account in the multivariate analysis were those known to be associated with asthma control in EGEA14 (online supplement) and another European cohort (ECRHS).12 In order to address the independent effect of each pollutant on asthma control, a two-pollutant model was performed, including O3-sum and PM10. Finally, analyses were also conducted on the three domains of asthma control (lung function, symptoms and exacerbations) (online supplement). The lack of data on activity limitation in our study did not allow including this domain in the asthma control classification. Analyses were performed with Stata 9.
Results
Of the 481 subjects included in the analysis, 44%, 29% and 26% had controlled, partly controlled and uncontrolled asthma, respectively. Asthma was more often uncontrolled in women (p=0.04) and in older subjects (p=0.003). Subjects with uncontrolled asthma used more inhaled corticosteroids (ICS) (p=0.001) (table 1). The IQR (defined by the difference between the third and the first quartiles) was 13 (25–38) μg/m3 for NO2, 11 (41–52) μg/m3 for O3-an, 13 (60–73) μg/m3 for O3-sum and 3 (18–21) μg/m3 for PM10. Annual and summer O3 measurements were highly correlated (Pearson coefficient r=0.72). NO2 was strongly negatively correlated with O3-an (r=−0.76) and moderately correlated with PM10 (r=0.45). O3-an and PM10 were not correlated (r=−0.08), and O3-sum and PM10 were weakly correlated (r=0.26).
Description of health covariates and pollutants for the total population and then defined by the level of control
Table 2 shows the results of the crude and adjusted associations between each of the air pollutants and asthma control. O3-sum and PM10 were positively associated with partly controlled and uncontrolled asthma, with a clear gradient from controlled, partly controlled and uncontrolled. For these two pollutants, the ORs assessed using the ordinal logistic regression were significant (ORs were 1.69 (95% CI 1.22 to 2.34) and 1.35 (95% CI 1.13 to 1.64) for O3-sum and PM10, respectively). Taking into account the family dependence of individuals did not change the coefficients (table 2). Adding the city of examination as a third level in the model decreased slightly the ORs for PM10 but not for O3-sum, and both associations remained significant (ORs were 1.69 (95% CI 1.21 to 2.36) and 1.33 (95% CI 1.06 to 1.67) for O3-sum and PM10, respectively).
Association between an IQR of each air pollutant and asthma control
In the two-pollutant model, asthma control estimates slightly decreased but remained statistically significant for both air pollutants. Using the ordinal logistic regression, the crude and adjusted ORs for those two pollutants included simultaneously in a unique model were 1.59 (95% CI 1.16 to 2.19) and 1.50 (95% CI 1.07 to 2.11) for O3-sum and 1.25 (95% CI 1.05 to 1.49) and 1.28 (95% CI 1.06 to 1.55) for PM10, respectively.
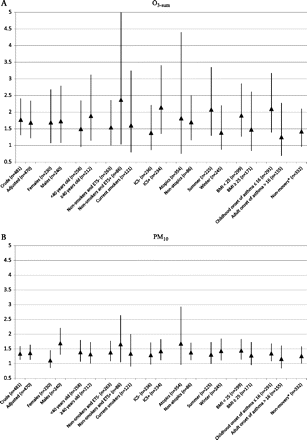
Figure 2A,B illustrates the adjusted ORs obtained from the ordinal logistic regression for O3-sum and PM10, respectively, stratified by sex, age, current active and passive smoking exposure, use of ICS in the past 12 months, atopy, season of examination, body mass index (BMI) and age of onset of asthma. The results from the sensitivity analysis only including subjects who had been living for at least 2 years in 2004 at their current address are also shown (thus is subjects who had not moved since 2002). All the estimates were above 1, and most of them were significant. No significant interaction was found, but the p value for interaction was borderline significant for sex and PM10 (p=0.06), suggesting that the association could be more significant in men.
Sensitivity analysis results. ORs and 95% CIs (from ordinal regression) showing the associations between each IQR of modelled O3-sum (A) and PM10 (B) and controlled asthma. *Non-movers are those who had been living for at least 2 years at the same address in 2004 (thus is those who have not moved since 2002). ICS: use of inhaled corticosteroids in the past 12 months.
The study of the associations between air pollution and each one of the three asthma control domains showed that increased levels of O3-sum and PM10 were associated with a lower FEV1. However, the association reached the significance level only for PM10 (p=0.002) (table 3). For the exacerbations, the ORs for O3-sum and PM10 were above 1. Higher exposure to O3-sum and PM10 were positively and significantly associated with higher symptom frequency (p=0.013 and p=0.002).
ORs derived from logistic regression between (a) lung function, (b) symptoms and (c) exacerbations and one IQR of each pollutant
Discussion
Long-term exposure to O3 and PM10 is associated with decreased asthma control in adults. This study is innovative since it shows for the first time that long-term exposure to air pollution is associated with uncontrolled asthma.
The associations observed in a large sample of well-characterised adults with current asthma were robust: (1) the associations concern the three domains of asthma control that were studied; (2) the associations were confirmed after adjustment on covariates including sex, age, BMI, smoking and ICS use; (3) the associations remained in a multilevel analysis taking into account the family and the city; and (4) the associations were not modified after stratification on several covariates. Due to the multidimensional approach of the control definition, the results increase the evidence of the combined acute and chronic respiratory effects of air pollution.
A major strength of EGEA is the detailed characterisation of subjects with asthma using epidemiologically defined criteria and extensive clinical characterisations in a country with homogeneous healthcare access. Phenotype misclassification is thus unlikely.17 The detailed EGEA2 questionnaire and examination of the subjects allowed the assessment of asthma control on the basis of the 2006–2009 GINA guidelines. However, EGEA had no information on activity restrictions. The lack of activity data may result in the misclassification of some of the subjects as having ‘controlled asthma’, resulting most likely in a loss of power of the effects of air pollution. In the ECRHSII study, 1032 patients were classified as having ‘current asthma’, and it was found that activity limitation had an impact on the asthma control level in 8.7% subjects (unpublished data). Thus, the lack of this dimension in the EGEA data is expected to impair asthma control classification in a few subjects only. We are aware that the current updated GINA guidelines now include aspects related to future risk, an aspect of importance from a clinical perspective, but difficult to assess clinically and impossible to include in epidemiological studies.
Another strength of our analysis is the robustness and consistency of the findings. For both pollutants (PM10 and O3), all estimates of the stratified analyses by sex, age, smoking, use of ICS, atopy, BMI, season of examination and age of onset of asthma were above 1 and in the same order of magnitude. Furthermore, both pollutants were associated with each of the three domains of asthma control, although the estimates were more stable and significant when using the integrated measure of asthma control. This indicates that results are not driven by one domain.
In the bi-pollutant model, the effect of both pollutants (O3-sum and PM10) remains, suggesting that the effect of both pollutants is independent. This may reflect different mechanisms by which each one affects asthma control. For example, O3 is a potent oxidant,19 ,20 while PM10 has oxidant properties and is an irritant producing local inflammation.20 ,21
One of the limitations of our study is the relatively large spatial scale of the air pollution models: 4 km2 grids. While this is appropriate for secondary or long-range pollutants with a relatively homogeneous distribution over longer distances (eg, O3 or PM10), this spatial scale cannot capture local traffic-related pollution as small-scale spatial contrasts are substantial for such pollutants.17 Models with higher spatial resolution have successfully used NO2 as a marker for local traffic-related pollutants, whereas our NO2 model fails to capture local conditions, resulting in large and most likely random misclassifications of exposure. This could explain why we do not find any association between NO2 and asthma control.
A limitation of our exposure assignment relates to the use of a model based on measurements taken in 2004 (yearly average), while asthma control data were collected between 2003 and 2007. However, spatial contrasts of median–range pollutants—relevant in our analyses—are unlikely to vary much from 1 year to another, although the absolute concentrations may differ across years.22 Results among subjects who had been living for at least 2 years in the same place as in 2004 were similar, indicating that this issue not to be of major relevance in our study.
Stressful living conditions may confound the observed associations if those correlate with asthma control and air pollution on the spatial scale used in our analyses. We do not have indicators of stress available; thus, a formal assessment of this concern is not possible. However, the multilevel analyses took into account ‘city’; thus, stressors associated with the city level would have been taken into account. The estimates were similar with and without control for city.
PM10 concentrations are quite low in the study. In France, before 2007, PM10 measurements were underestimated because the volatiles particles (such as the ammonium nitrate) were not considered. From 2007 (after the development of our map), the certified air quality monitoring agencies changed their measurement methods. The difference between the old and the new methods varied from 1.3- to 1.4-fold according to the characteristics of each region. Such scaling of the absolute levels does not affect our estimates of effects.
While there is an extensive literature about the acute effects of exposure to air pollution on different asthma control features, the long-term effects of exposure to air pollution on asthma control are unclear. Furthermore, most studies in asthma-air pollution were carried out in children. Hospitalisations or emergency visits for asthma were increased on days with higher air pollution concentrations.5 ,23 Fewer studies have found associations between acute exposure to traffic-related air pollution and lung function in asthmatics.24
To our knowledge, only two studies aimed to address the long-term effect of air pollution on some measures of asthma control. In a population-based study, living close to high traffic density roads was associated with poorer asthma control, assessed by more than one respiratory symptom per week or at least emergency department visit or hospitalisation in the last year.10 They found an association between O3 and asthma control only in the elderly and between PM10 and asthma control only in women.9 In their second study in adults, O3, PM10 and PM2.5 were significantly associated with symptoms but not with emergency department visits or hospitalisations.9 Our findings in which asthma control features were combined with lung function and the use of reliever treatment are in agreement with these studies.
In our study, the association of PM10 with asthma control seemed to be stronger in men. The existing literature about the difference of the air pollution effects according to sex is quite inconsistent, even if more studies report greater effects in women.25 As underlined recently, more studies are needed on this aspect to clarify gender differences and to assess the role of biological (ie, men have larger airways allowing larger pollutants such as PM10 to penetrate deeper), socio-cultural and exposure assessment factors (ie, women tend to spend more time at home), which may explain such differences.26
The association between O3 and PM10 and asthma control was not modified by smoking, the ICS use, atopy, BMI, age of onset of asthma and season, although we did observe some non-statistically significant differences in the point estimates for air pollution exposure between some of these groups. Larger studies may assess whether the apparently higher associations with pollution observed in some of these subgroups may be replicated, while we may conjecture about possible reasons for the observed but non-significant differences. In non-smokers exposed to environmental tobacco smoke (ETS), stronger associations of pollution with asthma control tended to be shown compared with smokers or non-smokers not exposed to ETS. This could be explained by the fact that ETS and air pollution share similar mechanisms of lung damage.26 ,27 In subjects using ICS, there is also a tendency for higher effects of air pollution, possibly reflecting a subgroup with more severe asthma that could be more susceptible to air pollution effects.28 In contrast to other findings, subjects with higher BMI are not more susceptible to air pollution in our data.29 However, most studies showing greater susceptibility of overweight subjects to air pollution were for cardiovascular outcomes. Estimates were also somewhat stronger among non-atopic subjects, while few studies reported atopic subjects to possibly react stronger to air pollution due to interactions between aeroallergens and pollutants.30 Estimates were also stronger in subjects with childhood asthma; this would suggest that air pollution affects more asthma control in subject who has been asthmatic for a long time. However, our study design is not tailored to address all these questions. The associations with O3 seemed to be higher for subjects examined during summer rather than winter. Reporting is most accurately recalled for the conditions during the past few weeks and months; thus, participants during summer provide more accurate data for the spring and summer. Furthermore, the exposure metric is the summer condition (O3-sum); therefore, this finding is expected.
The use of a composite asthma control classification is novel in air pollution research. Whereas air pollution was measured over 1 year, asthma control was assessed based on acute or subacute asthma events. In this study, the ‘long-term’ expression is used as opposed to day-to-day variations in the air pollutants concentrations. The models used to assess air pollution exposure were developed for the year 2004, which is a reasonable estimate of the longer term average air quality, given that the variation of annual means of background air pollutants is rather limited. An interesting question is whether ‘asthma control’ reflects a summary of the acute effect of air pollution on asthma and how it relates to the chronic development and worsening of the disease. As shown in general populations, a single measure of lung function may be affected independently by the level of pollution on the day of the examination and the long-term exposure.31 ,32 To formally disentangle the chronic from the acute effects of air pollution requires different study designs and estimates of daily levels of air pollution that were not available in our population. Results support an effect of long-term exposure to air pollution on asthma control in adulthood in subjects with pre-existing asthma. It remains possible, however, that as suggested for occupational asthmogens, environmental insults may also induce directly a more severe form of disease.33 Increasing evidence suggest that environmental exposures play a role at the various steps of the disease process. However, whether long-term exposure to air pollution is at the same time a cause for the development of the underlying chronic asthma pathology is subject to investigations and cannot be addressed with the data used in this analysis.
In summary, our results indicate that both ambient O3 and PM10 concentrations jeopardise asthma control in adults. The results are robust. The clinically relevant concept of ‘asthma control’ with its integrated assessment of acute and chronic dimensions of the disease offers novel ways to quantify and monitor the public health relevance of air pollution among adults with asthma.
What is already known on this subject
Acute exposure to air pollution is associated with adverse respiratory effects in adults with asthma, such as the increase in emergency visits, exacerbations, medication intake and symptoms.
However, the effect of long-term exposure to air pollution on asthma control, assessed by combining several domains of control reflecting both acute and chronic respiratory conditions, has never been studied.
What this study adds
Our results suggest a robust association between long-term exposure to O3 and PM10 and uncontrolled asthma, defined according to the international clinical guidelines and combining three different domains (lung function, symptoms and exacerbations), in a large number of well-characterised current asthmatics from the Epidemiological study on Genetics and Environment of Asthma.
Acknowledgments
The authors thank N. Jeannée from Géovariances and all those from the French Institute for Environment, and particularly M. Ba, for their work on the geospatial models.
References
Footnotes
EGEA cooperative group. Coordination: F Kauffmann; F Demenais (genetics); I Pin (clinical aspects). Respiratory epidemiology: Inserm U 700, Paris M Korobaeff (EGEA1), F Neukirch (EGEA1); Inserm U 707, Paris: I Annesi-Maesano; Inserm CESP/U 1018, Villejuif: F Kauffmann, N Le Moual, R Nadif, MP Oryszczyn; Inserm U 823, Grenoble: V Siroux Genetics: Inserm U 393, Paris: J Feingold; Inserm U 946, Paris: E Bouzigon, F Demenais, MH Dizier; CNG, Evry: I Gut, M Lathrop. Clinical centers: Grenoble: I Pin, C Pison; Lyon: D Ecochard (EGEA1), F Gormand, Y Pacheco; Marseille: D Charpin (EGEA1), D Vervloet; Montpellier: J Bousquet; Paris Cochin: A Lockhart (EGEA1), R Matran (now in Lille); Paris Necker: E Paty, P Scheinmann; Paris-Trousseau: A Grimfeld, J Just. Data and quality management: Inserm ex-U155 (EGEA1): J Hochez; Inserm CESP/U 1018, Villejuif: N Le Moual, Inserm ex-U780: C Ravault; Inserm ex-U794: N Chateigner; Grenoble: J Ferran.
Funding The EGEA study was supported in part by grants from Merck Sharp & Dohme (MSD); Hospital Program of Clinical Research (PHRC)-Paris; National Research Agency—health environment, health work program; National Research Agency (ANR)—Biological collections for health program; French Agency of Health, Safety, Environment and Work (AFSSET), Agence de l'Environnement et de la Maitrise de l'Energie (ADEME), the National Scientific Committee of the Medico-technology support at home (AGIR à dom) and the Isere Committee against Respiratory Diseases (COMARES).
Competing interest None to declare.
Patient consent Obtained.
Ethics approval This study was conducted with the approval of the Cochin Royal Hospital, Paris, for the first survey (EGEA1); Necker Enfants Malades Hospital, Paris, for the second survey (EGEA2).
Provenance and peer review Not commissioned; externally peer reviewed.