Article Text
Abstract
Hypoxia is common in many chronic lung diseases. Beyond pulmonary considerations, delivery of oxygen (O2) to the tissues and subsequent O2 utilisation is also determined by other factors including red blood cell mass and iron status; consequently, disruption to these mechanisms provides further physiological strains on an already stressed system. O2 availability influences ventilation, regulates pulmonary blood flow and impacts gene expression throughout the body. Deleterious effects of poor tissue oxygenation include decreased exercise tolerance, increased cardiac strain and pulmonary hypertension in addition to pathophysiological involvement of multiple other organs resulting in progressive frailty. Increasing inspired O2 is expensive, disliked by patients and does not normalise tissue oxygenation; thus, other strategies that improve O2 delivery and utilisation may provide novel therapeutic opportunities in patients with lung disease. In this review, we focus on the rationale and possibilities for doing this by increasing haemoglobin availability or improving iron regulation.
- Systemic disease and lungs
- COPD ÀÜ Mechanisms
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background
Humans require oxygen (O2) delivery to core organs (eg, heart, brain or kidneys) at rest and, when exercising, oxygen must also be delivered to exercising muscle if anaerobic metabolism is to be prevented. Chronic respiratory diseases may impair O2 delivery at multiple levels. For example, in chronic obstructive pulmonary disease (COPD), the problem is often predominantly one of respiratory mechanics, whereas in lung fibrosis, it may be oxygen transport across the alveolar capillary junction. Both these and other conditions may also be associated with pulmonary hypertension and impaired cardiac output, particularly during exercise. Patients with chronic respiratory diseases need to increase respiratory drive1 to maintain O2 delivery irrespective of the aetiology of the deficiency, which gives rise to the symptom of breathlessness which in turn leads to reduced physical activity with an adverse effect on quality of life. When disease is sufficiently severe, this effect is common to several different respiratory populations, especially COPD, asthma, pulmonary fibrosis and bronchiectasis.
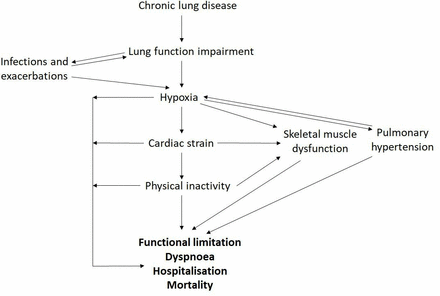
Delivery of O2 to the tissues is also determined by non-respiratory factors including red blood cell mass, tissue capillarity and cardiac output. Utilisation of O2 also fundamentally drives tissue O2 requirements and iron plays a key role through mediating cellular respiration and energy metabolism (figure 1). Disruption to these mechanisms may trigger a downward cycle in which hypoxia imposes further physiological strains on an already stressed system. Deleterious effects of hypoxia through the multiple downstream effects of poor tissue oxygenation include increased cardiac strain, pulmonary hypertension and salt and water imbalance mediated through the impact of cardiac and circulatory insufficiency (Cor Pulmonale). Pathophysiological secondary damage to multiple other organs and tissues including skeletal muscle, brain, kidney and gut are also attributable to impaired O2 delivery.2 3 In the setting of relative tissue hypoxia and an imbalance between oxygen demand and respiratory capacity, a ‘spiral of decline’ ensues resulting in progressive deconditioning, frailty and both enhanced oxygen demand and increased respiratory loading through premature exercise induced acidosis (figure 2).4 A multimodal approach to managing patients with chronic lung disease is advocated,5 comprising approaches aimed at enhancing respiratory capacity (eg, bronchodilation or lung volume reduction in emphysema) and reducing respiratory demand (such as exercise training). Novel approaches that impact upstream pathophysiological events including impaired tissue oxygen delivery may be expected to provide significant therapeutic benefit.6
The role of iron and red cell mass in tissue oxygen availability and utilisation.
The ‘spiral of decline’ in chronic lung disease: an interplay between pathophysiological features.
The availability of O2 is essential for organ viability and function and is subsequently closely regulated at a cellular level and through integrated physiological responses. Although a significant proportion of subjects with severe lung disease exhibit O2 desaturation at rest, tissue oxygen delivery is further impaired under conditions of strain, for example, exercise7 or during exacerbation. It is clear that routine clinical measures of O2 delivery such as fingertip haemoglobin (Hb) oxygen saturation (SpO2) may inappropriately provide reassurance and significantly underestimate the degree of impairment of tissue oxygenation.8 For example, while approximately half of patients with COPD with resting SpO2≤95% desaturate on exertion, an additional 16% desaturate on exercise despite having resting SpO2>95%.9 Furthermore, 38% of patients with moderate to severe COPD desaturate at night without evidence of obstructive sleep apnoea10 due to physiological reduction of neural drive.11
Hb, which transports O2 in the blood, has a fundamental role in O2 delivery because the solubility of O2 is low; at a normal partial pressure of oxygen (PaO2) of 13 kPa, there is only 3 mL of dissolved O2 per litre compared with 200 mL per litre bound to Hb.3 Thus, quantitatively, anaemia may have a greater impact on oxygen delivery than reduced O2 saturation (figure 3). Subsequently, tissue O2 availability may be reduced even when O2 content measured by traditional methods, such as PaO2 or SpO2 is adequate.
The comparative oxygen content of circulating blood within the setting of physiologically relevant anaemia and reduced Hb oxygen saturation. In a patient with an Hb of 11 g/dL and oxygen saturations of 88%, increasing the Hb by 1 g/dL has double the impact on oxygen content than improving Hb oxygen saturation by 4%. Improvements in oxygen content may occur with increased Hb even in the setting of normal saturations (eg, as may be relevant in concomitant cardiac failure given that oxygen delivery is the product of cardiac output and oxygen content).
It has been shown in COPD that Hb is directly related to oxygen consumption (VO2 max) even after accounting for disease severity12 and, at a patient level, symptomatic burden and mortality.13 14 Indeed O2 availability impacts ventilation, pulmonary blood flow and gene expression throughout the body.2 Long-term oxygen therapy (LTOT) is an established therapy that reduces both morbidity15 and mortality16 17 in severely hypoxic patients and, in appropriate populations, supplementing inspired O2 increases exercise duration.18 Scenarios where other factors may be more important than traditional measures of oxygenation and consistent with the relevance of Hb concentration to circulating O2 content (figure 3) include patients with less severe exercise O2 desaturation not benefitting from supplementing inspired oxygen19 20 and conflicting data surrounding the impact of O2 supplementation on exercise performance and health-related quality of life (HRQoL).21
Despite the benefits of O2 supplementation, even when the recipient is carefully selected, there remain the practical issues in ensuring that inspired O2 is supplemented for the required time (>15 hours a day) to achieve benefit. Furthermore, in some subsets such as ongoing smokers, the risks may actually outweigh the benefits.22 23 Given the challenges of normalising O2 delivery in patients with established lung disease, other strategies that improve O2 content and metabolism might deliver direct patient benefits and synergise with the benefits of other available therapeutic strategies including oxygen supplementation itself. Thus, increasing Hb availability represents a novel therapeutic opportunity in this population24 with the potential to improve exercise tolerance, dyspnoea, fatigue and HRQoL, and in the longer term, reduce the risk of hospitalisation and mortality (figure 2). Beyond any relevance to erythropoiesis, there is also a potential role for treating dysregulated iron metabolism in chronic lung disease, given a shared pathophysiology, hereon, we detail the aetiology and relevance of anaemia and functional iron deficiency in respiratory disease.
The clinical relevance of anaemia, inflammation and dysregulated iron metabolism
Many chronic diseases have been shown to impact haematopoiesis and this is especially relevant in chronic lung disease. Polycythaemia has traditionally been considered an indication for venesection in COPD given the potential risks of hypercoagulability; however, this erythropoietic response is driven by tissue hypoxia and following the institution of LTOT as a standard of care, polycythaemia is now three times less prevalent than anaemia.14 In cystic fibrosis (CF), pulmonary arterial hypertension (PAH) and COPD, a significant number of subjects have anaemia and or iron deficiency (circa 20%–40%),14 25–30 although this area is poorly studied and the incidence may be significantly underestimated, since establishing iron deficiency is complicated because of the effects of inflammation on serum iron, transferrin and ferritin. Contextually, a role for functional iron deficiency may be equally relevant as may be disease-specific considerations including propensity to infection.30 Although not currently part of routine clinical assessment, serum transferrin receptor levels appear to be unaffected by inflammation and may more accurately reflect iron status in chronic lung disease; circulating levels in the normal range reflect erythropoietic activity and levels are disproportionately elevated in iron deficiency.31
In a series that considered this complexity, anaemia of chronic disease or ‘anaemia of inflammation’, was observed in 10% of patients with stable COPD.12 Congruent with the pathophysiological relevance of inflammation, the prevalence of anaemia in COPD has been observed to be up to 44% among patients admitted with acute exacerbation25 and the presence of anaemia in this scenario is independently associated with mortality.26 In fact, anaemia and/or iron deficiency have been consistently shown to have a strong correlation with morbidity12 27 32 and mortality across several lung diseases,13 14 33–35 the reported prevalence has been widely variable due to the different characteristics of study populations and the lack of a consensus definition of anaemia in the context of chronic lung disease. While most authors use the WHO definition (<13 g/dL for males and 12 g/dL females), some use the same Hb level for both men and women (since most women are postmenopausal in these populations), while others have used haematocrit as the identifying factor. Although the prevalence also varies across the various respiratory populations, the impact of anaemia in the setting of impaired physiology and symptomatic limitation is expected to be common to a range of respiratory diseases.
Consistent with the physiological relevance of anaemia, several clinical studies in COPD have reported a significant deleterious effect of anaemia on exercise capacity, with anaemic patients being more dyspnoeic and exhibiting functional exercise limitation12 14 36 and reduced maximal O2 consumption.12 In a regression analysis, higher Hb levels were independently associated with improved exercise capacity and HRQoL.37 Improved Hb is expected to benefit O2 delivery even beyond the lower limit of the normal Hb range (figure 3) and in fact higher Hb levels (14.3 g/dL in females, 15.1 g/dL in males) have been shown to be associated with improved survival in patients with chronic respiratory failure.33 The performance benefits of elevating Hb above the physiological range are well established in athletes and this is expected to translate to respiratory patients who are compromised in their ability to establish appropriate ventilatory and cardiac responses to exercise.38 39 Although iron deficiency does not appear to impact viscosity, higher haematocrit has been associated with hyperviscosity in other populations where the association between higher haematocrit and greater exercise capacity is maintained despite this.40
Mechanisms of anaemia in chronic lung disease
The mechanisms leading to anaemia in chronic lung disease are complex and have not yet been fully characterised. Factors pertinent to ageing in general and to other populations appear to be implicated in the process; however, in the context of anaemia of chronic disease, the underlying inflammatory disease and erythropoietin (EPO) response seem the most relevant (figure 4). Inflammation in lung disease is thought to play a major role through both direct and indirect mechanisms. Inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-10, IL-22, interferon-γ and tumour necrosis factor 1-α interfere with the normal function of several molecules involved in iron metabolism.41 42 These inflammatory markers are generally increased in anaemic patients with chronic lung disease and are associated with reduced serum iron levels and decreased iron availability for erythropoiesis;36 43 they are believed to do this through the destruction of red blood cell precursors and decreasing the number of EPO receptors on progenitor cells.44 Interestingly, this increase in inflammatory cytokines, particularly IL-6, is also considered to be an innate immune response to infection as it reduces iron availability for the growth of extracellular pathogens and is relevant to respiratory populations predisposed to bacterial and viral infections.42 Beyond the direct effects on iron metabolism, inflammatory cytokines have been found to strongly influence hepcidin production under conditions of hypoxia.45 Hepcidin production is normally reduced in response to hypoxia and anaemia so that erythropoietic iron demands can be fulfilled; however, in chronic inflammatory states, IL-6 induces synthesis and secretion of hepcidin by hepatocytes and consequently reduces extracellular iron availability.46 47 These different underlying causes of anaemia conferred by chronic systemic inflammation are seen in a variety of conditions, thus impairing normal corrective mechanisms and they are also relevant in chronic lung disease (figure 4).
The clinical and biological factors relevant to iron dysregulation and anaemia of chronic disease in respiratory populations. EPO, erythropoietin.
Other factors besides inflammation have been described as being relevant in the development of anaemia in chronic lung disease including: (1) renin-angiotensin-aldosterone system dysregulation resulting in reduced erythropoiesis,48 49 (2) renal impairment resulting in reduced EPO production and reduced hepcidin clearance,50 51 (3) theophylline treatment resulting in reduced EPO production and/or direct inhibition of erythroid progenitor cells,52 53 (4) androgen deficiency resulting in reduced erythropoiesis54 and (5) malnutrition resulting in iron, vitamin B12 and folic acid deficiencies.55 56 Other factors relevant to general populations such as impaired bone marrow response may also be relevant, especially in COPD and idiopathic pulmonary fibrosis (IPF) which typically occur in older populations.55
EPO acts as the primary stimulus for erythropoiesis and is normally produced by the kidneys in response to reduced tissue oxygenation. Renal impairment, which is common in patients with COPD, can lead to disruption in EPO production. However, in COPD, the relationship between hypoxia, inflammation, iron and EPO is complex with studies showing differing results. Non-clinical and clinical evidence suggests that EPO production could be inhibited by the effects of inflammatory cytokines;57 however, some clinical studies have also identified mildly increased levels of EPO (circa twofold) in COPD.58 59 While upregulation of EPO is expected in the setting of hypoxia, these increased EPO levels in COPD are not necessarily translated to enhanced erythropoiesis. While some have considered this as EPO resistance, in the setting of multiple factors relevant to anaemia in this population, especially inflammation, this may be alternatively considered a relative EPO deficiency (figure 4);57 the levels observed are certainly well below those seen when a functional response occurs—in the setting of erythropoiesis therapeutically driven by exogenous EPO, EPO levels may be 100s of fold greater.60 61 Consistent with an inadequate EPO response in chronic lung disease, exacerbations which are inherently associated with inflammation and hypoxia, are associated with an increased prevalence of anaemia25 and EPO levels have been demonstrated to fall in this setting.62
Iron therapy in clinical practice
In COPD, patients with iron deficiency are more hypoxaemic even though they do not have significantly worse airflow limitation.63 Beyond erythropoiesis, a role for iron replacement in lung disease is supported by the role iron has in various processes including its fundamental role in mitochondrial respiration and energy processing, a structural role in proteins such as myoglobin and in the response to infection. It has been demonstrated that iron deficiency results in exaggerated hypoxic pulmonary hypertension that is reversed by subsequent iron administration64 65 and studies evaluating the potential benefit of iron replacement in PAH are currently running. Although routinely used for iron replacement, oral iron supplementation is limited by absorption and gastrointestinal side effects. Intravenous iron is an established therapy in heart failure and continues to be studied in various populations. The FAIR-HF Trial,66 67 a multicentre, randomised, double-blind, placebo-controlled trial of intravenous ferric carboxymaltose for symptomatic patients with heart failure and iron deficiency anaemia, found a 1 class improvement in New York Heart Association was 2.40-fold more likely to occur (95% CI 1.55 to 3.71) in the treatment group which demonstrated a change in 6-min walk test distance of 35±8 m, p<0.001 at week 24. Similar results were obtained from the CONFIRM-HF study.68 In COPD, there are presently no reported data from randomised controlled trials investigating the effect of either oral or intravenous iron, but two studies evaluating intravenous iron are presently recruiting according to clinicaltrials.gov (NCT NCT03050424 and NCT02416778). In respiratory populations, iron deficiency has been associated with reduced aerobic capacity and an impaired response to pulmonary rehabilitation in COPD,69 and mortality in the setting of tuberculosis infection.35
We are not aware of any trials investigating iron replacement in patients with IPF or bronchiectasis, although there have been small studies in CF where we note that iron chelation has been proposed as a therapeutic option in patients colonised with Pseudomonas aeruginosa through a proposed beneficial effect on Pseudomonas cell-cell communication.70 71 As in COPD, in the setting of inflammation, an impaired erythropoietic response to hypoxaemia has been described in adult patients with CF.27 In one study, a 3-month course of oral iron did not increase Hb in a subgroup of patients with CF and functional iron deficiency. Absorption and compartmentalisation of iron are likely to be relevant and this concept is supported by a very small case series of adult patients with CF, who when given intravenous iron for anaemia refractory to oral iron, demonstrated a significant rise in Hb concentration and mean corpuscular volume within days of therapy.72 These findings are consistent with suppression of iron absorption by hepcidin. Although commonly used in clinical practice and generally regarded as safe, exogenous iron compartmentalising to the tissues and contributing to oxidative stress-related tissue damage may be a concern in chronic lung disease.73
Novel treatment options
The only disease area where correction of anaemia of chronic disease has been extensively studied is in chronic kidney disease (CKD) in response to recombinant human erythropoietin (rhEPO) and its analogues. Even in the presence of normal lung physiology, therapy in CKD has been demonstrated to improve exercise tolerance, HRQoL, cerebration and left ventricular hypertrophy.74 There is also evidence to show a beneficial effect in chronic heart failure with improved exercise tolerance, decreased oxygen utilisation on exercise, improved renal function, decreased brain natriuretic peptide and reduced hospitalisation;75 however, concerns over the deleterious effects of increased EPO levels and adverse cardiovascular events have led to a lack of enthusiasm for use of EPO in chronic heart failure and more conservative usage in CKD.74 76 Treatment of anaemia of CKD with rhEPO is associated with increased cardiovascular risk which is postulated to be related to the associated increases in EPO exposure.77 It is unclear if this risk can be attributed solely to EPO exposure as opposed to targeted or achieved Hb, the specific relevance of the absolute Hb, Hb change, the rate of Hb change or the EPO levels that occur in dosing patients with rhEPO. Given that normal Hb levels are protective and other therapies increase Hb without risk, the supraphysiological levels of EPO and impact on sympathetic drive seem inherently most relevant but this is as yet unproven. Subsequently, for CKD, US Prescribing Information for all currently approved erythropoietin stimulating agents (ESAs) contains a Boxed Warning and European labels contain warnings that patients experienced greater risk for death, serious adverse cardiovascular reactions and stroke when administered ESAs to target a Hb level of greater than 11 g/dL.61
While ESAs have not been generally used in lung disease outside the setting of significant concomitant renal disease, iron therapy is commonly used. Furthermore, Hb in the normal range is associated with better survival and reduced breathlessness, improved exercise performance and HRQoL. It is expected that anaemic patients with chronic lung disease will benefit from the potential multimodal benefits of having improved Hb levels and improved O2 delivery to the tissues and that in this context treating anaemia of chronic disease in patients with chronic lung disease is a different paradigm to other diseases, especially when it is considered that beyond iron replacement, there are no established relevant treatment options in these patients. Few interventional studies have been conducted to assess the treatment of anaemia in chronic lung disease. Several small studies have been conducted which have demonstrated the potential benefit of this approach with reduced ventilatory requirements following red blood cell transfusion to increase Hb from 9.8 to 12.3 g/dL78 and successfully weaning patients from ventilatory support when their Hb values were increased to within the normal range (12 g/dL).79 A small uncontrolled study demonstrated that intravenous iron and ESA resulted in increases in Hb which directly correlated with improvements in dyspnoea in anaemic patients with COPD.80 Although it is not possible to differentiate the effect of iron and ESA in the latter study, overall, these studies suggest a direct correlation between improvements in Hb and physiological parameters through improved circulating oxygen content in patients with chronic lung disease which should lead to symptomatic benefit and potentially impact longer term outcomes. Indeed, it is possible that the potential benefits to be derived from improved Hb (through oxygen delivery) and iron regulation (through improved mitochondrial function and pulmonary vascular tone) may be additive.
Small molecules which inhibit hypoxia-inducible factor (HIF) prolyl-4-hydroxylases, preventing the breakdown of the HIF transcription factor, are currently in development. In preclinical studies, HIF stabilisation results in the accumulation of HIF and upregulation of HIF-responsive genes.61 This biological activity simulates multiple components of the natural tissue response to hypoxia, including erythropoiesis, angiogenesis, immune modulation and enhanced cell survival. Agents targeting HIF stabilisation have been shown to induce endogenous EPO production and improve markers of iron metabolism, resulting in erythropoiesis and increased Hb levels in subjects with anaemia associated with CKD81 and also in other populations such as patients with peripheral arterial disease.82 These molecules may be of value in chronic lung disease where a dysregulated EPO response may revert to a more functional response in the setting of improved longer term iron regulation through HIF stabilisation. Through this mechanism and improved tissue O2 delivery, chronic EPO levels may be lower and more stable in addition to more functional. Investigation of agents targeting HIF-PHIs (prolyl hydroxylase inhibitors) for the treatment of anaemia associated with CKD have progressed into Phase III clinical studies, including large cardiovascular outcome trials. For the treatment of anaemia of CKD, HIF stabilisation has the potential to provide similar or better efficacy to approved therapies with the potential for an improved safety profile, most notably compared with rhEPO and conventional ESAs. Adverse effects that were initially observed with ESAs included elevated blood pressure, seizures and a high rate of thrombotic events, although approaches to slowly increase the haematocrit seem to minimise these problems, concerns around the development of immune reactions to developing antibodies and cardiovascular events remain.83 The risks associated with rhEPO may hypothetically be found with HIF-PHIs since they also raise Hb and EPO levels, although the mechanism of action of increasing red blood cell mass is not solely mediated through EPO, and EPO is not raised to the supraphysiological levels observed following administration of exogenous rhEPO injection. Hypothetical risks for malignancy and pulmonary hypertension have been suggested by findings in patients with naturally occurring mutations that result in HIF upregulation and these may be particularly relevant in chronic lung disease where patients are already predisposed to these afflictions.83 84 Furthermore, beyond the relevance of iron in innate immunity, animal and cell studies indicate a potential for HIF upregulation to increase the risk of infection.85–87 Despite these considerations, HIF-PHIs have been considered safe and well tolerated in clinical studies thus far and outcome studies currently underway will add further to the longer term safety experience with manipulating HIF as a therapeutic strategy.81 Given pulmonary hypertension may deteriorate under strain in chronic lung disease, this requires further consideration. The key driver for the development of pulmonary hypertension in chronic lung disease is hypoxia, subsequently improved O2 delivery (through improved Hb) and improved iron availability mediated by HIF stabilisation could potentially ameliorate pulmonary hypertension. Anaemia in the setting of pulmonary hypertension and COPD is associated with a greater rate of deterioration in exercise capacity, hypoxaemia, quality of life and acute exacerbations.88 Recent clinical experience indicates that HIF-PHI can increase EPO levels at very small doses where drug exposure may be largely or even entirely confined to the liver and kidneys (EPO-producing organs).61 83 Therefore, it is possible the EPO-producing potential of these agents can be realised without direct effects on the lung vasculature.
Given the experience with ESAs, there may be reservations about targeting Hb levels>11 g/dL; however, considering physiological and clinical differences, it may not be appropriate to extrapolate considerations from other populations to patients with chronic lung disease. As with CKD, patients with chronic lung disease are predisposed to increased CV morbidity and mortality. The current limits (to not start rhEPO unless Hb<10 g/dL) with rhEPO treatment in CKD where there is not a primary driver for hypoxia beyond the anaemia itself make development of rhEPO in the chronic lung disease population challenging given that most of the population are not anaemic.14 89 In this regard, it is relevant that the benefit:risk considerations in chronic lung disease are different from CKD, given the key synergistic impacts of impaired pulmonary gas exchange and O2 carriage in this population where there is no established treatment option for anaemia of chronic disease. Clinical studies have demonstrated that treatment with HIF-PHIs is associated with plasma EPO levels that remain within the physiological range for the CKD patient population,90 and hence risks associated with supraphysiological EPO levels may be significantly lower. Improved O2 content through improved Hb may improve cardiovascular outcomes in chronic lung disease at higher Hb levels, given the increased cardiac risk, especially during periods of strain such as hospitalisations that are associated with hypoxia and an increased risk of anaemia.91 Consistent with the variable impact of impaired O2 delivery through reduced Hb in different populations, despite potential safety concerns in CKD,92 a meta-analysis has demonstrated a reduction in hospitalisations in chronic heart failure93 in the absence of any mortality benefit with rhEPO in this population.94
In conclusion, anaemia and dysregulated iron metabolism are prevalent in chronic lung disease, and in the setting of impaired O2 delivery in patients who have a limited ability to compensate, there is associated morbidity and mortality. Therapies used in other disease areas may have particular benefits in this population. Given changes in standards of care, clinical features in chronic lung disease are evolving and newer therapies may provide opportunities to deliver improved outcomes in chronic lung disease. Given differences in populations and the ongoing morbidity and mortality burden, studies in chronic lung disease are warranted to confirm any benefit to treating anaemia and dysregulated iron metabolism and to mitigate any theoretical safety risks that extend from the literature and other populations.
Acknowledgments
The authors would like to thank Professor Sir Peter Ratcliffe (Nuffield Department of Medicine, Oxford) for reading and commenting on this State of the art review.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
Footnotes
SJP and MIP contributed equally.
Contributors The review was first drafted and developed by MSP and SJP with subsequent contribution from all authors.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer The views expressed by Elizabeth McKie, an employee of GlaxoSmithKline, are her own and may not necessarily express or represent the opinions of GlaxoSmithKline.
Competing interests MSP is an AstraZeneca employee and former GlaxoSmithKline (GSK) employee, EM is a GSK employee and shareholder and SJP is a GSK shareholder and a Sanofi employee. MCS reports personal fees from GSK, Boerhinger Ingelheim and Nutricia outside of the submitted work and MIP has received grants from GSK outside of the submitted work.
Patient and public involvement statement There has not been any patient involvement as there are no unpublished patient data in this review article.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; internally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as supplementary information.