Article Text
Abstract
Background There is increasing evidence that vitamin D (VD) deficiency may increase individuals’ risk of COVID-19 infection and susceptibility. We aimed to determine the relationship between VD deficiency and sufficiency and COVID-19 seropositivity within healthcare workers.
Methods The study included an observational cohort of healthcare workers who isolated due to COVID-19 symptoms from 12 May to 22 May 2020, from the University Hospitals Birmingham National Health Service Foundation Trust. Data collected included SARS-CoV-2 seroconversion status, serum 25(OH)D3 levels, age, body mass index (BMI), sex, ethnicity, job role and comorbidities. Participants were grouped into four VD categories: (1) Severe VD deficiency (VD<30 nmol/L); (2) VD deficiency (30 nmol/L ≤VD<50 nmol/L); (3) VD insufficiency (50 nmol/L ≤VD<75 nmol/L); (4) VD sufficiency (VD≥75 nmol/L).
Results When VD levels were compared against COVID-19 seropositivity rate, a U-shaped curve was identified. This trend repeated when participants were split into subgroups of age, sex, ethnicity, BMI and comorbidity status. Significant difference was identified in the COVID-19 seropositivity rate between VD groups in the total population and between groups of men and women; black, Asian and minority ethnic (BAME) group; BMI<30 (kg/m2); 0 and +1 comorbidities; the majority of which were differences when the severely VD deficient category were compared with the other groups. A larger proportion of those within the BAME group (vs white ethnicity) were severely VD deficient (p<0.00001). A larger proportion of the 0 comorbidity subgroup were VD deficient in comparison to the 1+ comorbidity subgroup (p=0.046).
Conclusions Our study has shown a U-shaped relationship for COVID-19 seropositivity in UK healthcare workers. Further investigation is required to determine whether high VD levels can have a detrimental effect on susceptibility to COVID-19 infection. Future randomised clinical trials of VD supplementation could potentially identify ‘optimal’ VD levels, allowing for targeted therapeutic treatment for those at risk.
- COVID-19
- respiratory infection
- viral infection
Data availability statement
Data are available upon reasonable request. The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Vitamin D has numerous mechanistic roles within the immune system and there is increasing evidence to suggest a role of VD in susceptibility to COVID-19 infection.
VD deficiency (<30 nmol/L) has been shown to be an independent risk factor for development of COVID-19 seroconversion in UK healthcare workers.
WHAT THIS STUDY ADDS
This study shows that there is a U-shaped curve relationship of COVID-19 seropositivity and levels of VD deficiency/sufficiency in symptomatic healthcare workers.
The U-shaped curve persisted when subcategorising the participants according to baseline demographics but was most pronounced in the black, Asian and minority ethnic group.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study shows a signal that higher VD levels could also have a detrimental effect on COVID-19 susceptibility, which requires further investigation.
Future randomised clinical trials of VD supplementation are needed to identify ‘optimal’ VD levels to allow for targeted therapeutic treatment for at-risk groups.
Introduction
Vitamin D (VD) is an essential lipophilic secosteroid that has a complex interrelationship with both the innate and adaptive immune system.1 Vitamin D3 deficiency (VDD) has been shown to be associated with the increase of infection,2 which may be due to changes in functional immunity.3 In bacterial sepsis, VD deficiency has been shown to have a role in the development of acute respiratory distress syndrome (ARDS), a complication also seen in severe COVID-19 infection.4 As an estimated 39% of patients with COVID-19 with ARDS have died,5 it is vital to understand whether VD deficiency increases the risk of SARS-CoV-2 infection.
Individuals of black, Asian and minority ethnic (BAME) groups appear to be disproportionately at risk of COVID-19.6 VD deficiency is more prevalent in darker-skinned individuals in comparison with those of lighter skin.7 This may in part provide some explanation for the increased risk of COVID-19 infection in those of BAME ethnicity, and whether addressing lower serum levels could reduce infection risk.
A recent review which collated association of VD with both COVID-19 infection and mortality found mixed results.8 There was an overall increased risk of infection and mortality in those who were VD deficient, but a large proportion of studies did not control for important confounders. There is limited data on VD levels and associated COVID-19 in healthcare workers, who were at a higher risk of developing COVID-19 during the pandemic.9 We published a rapid research letter during the pandemic of a cross-sectional study of UK healthcare workers who isolated due to symptoms of COVID-19.10 Our early analysis found that BAME ethnicity are at the highest risk of VD deficiency (VD levels <30 nmol/L) and that VD deficiency was an independent risk factor for development of COVID-19 seroconversion; the biggest differences in seroconversion were seen in the BAME male group. However, the association of differing degrees of deficiency and relationship with demographics, comorbidity and COVID-19 infection were not investigated.
This study aims to determine in detail the relationship between VD deficiency and sufficiency and COVID-19 infection in a cohort of healthcare workers who isolated due to symptoms of COVID-19.
Methods
As part of an observational study, healthcare workers were recruited from 12 May to 22 May 2020 from the University Hospitals Birmingham National Health Service Foundation Trust (UHBFT). This was part of the COVID-19 Convalescent Immunity Study. The inclusion criteria of this cohort studied were staff members who had symptoms suggestive of COVID-19. Demographic details were obtained including age, body mass index (BMI), sex, ethnicity and comorbidities. Blood samples were taken to the laboratory for processing to obtain serum for SARS-CoV-2 antibody and for the VD assay at the same time point. The median time from symptom onset to sample collection was 48 days.10
Anti-SARS-CoV-2 spike glycoprotein antibodies were measured using a combined IgG, IgA, IgM ELISA antibody with 98.3% (95% CI 96.4% to 99.4%) specificity and 98.6% sensitivity (95% CI 92.6% to 100%) (product code MK654, The Binding Site, Birmingham).11 Seroconversion was used as an immunological surrogate of prior SARS-CoV-2 infection.
VD status was determined by measurement of serum 25(OH)D3 using mass spectrometry. In this method serum samples were subjected to a protein crash followed by online extraction and 25(OH)D3 was quantified using liquid chromatography- mass spectrometry (LC-MS/MS) (Shimadzu ultra performance liquid chromatography (UPLC) system) with an AB SCIEX Triple Quad 4500 mass spectrometer. Concentrations of VD were reported in nmol/L and stratified into the following categories: (1) Severe VD deficiency (VD<30 nmol/L); (2) VD deficiency (30 nmol/L ≤VD <50 nmol/L); (3) VD insufficiency (50 nmol/L ≤VD <75 nmol/L); (4) VD sufficiency (VD ≥75 nmol/L).12
Data were analysed using IBM SPSS Statistics (V.27). Continuous data were reported as mean±SD or median-IQR depending on the normality of distribution as assessed using the Shapiro-Wilk test. Categorical data were reported via frequency and proportion. When VD was stratified into groups, continuous variables were compared using either independent samples t-test or Mann-Whitney U test. Categorical data were assessed with Fisher’s exact test. Correlation between VD and SARS-CoV-2 seropositivity were determined by a second-order polynomial regression. Statistical significance was defined as a value of p<0.05 in all cases. Graphs were created using Excel (V.2202) from MS office for enterprise.
Patient and public involvement
This study involved a cohort of healthcare workers who were recruited through open invitation via UHBFT email to all staff and advertised via social media. The study was an United Kingdom Research and Innovation (UKRI) urgent public health badged study and due to the need for rapid recruitment no patient and public engagement was undertaken prior to the initiation of the study.
Results
In total, there were 379 participants. The median age of the cohort was 42.0 (IQR 30.0–50.0) years. Representation from the cohort included 282 women (74.4%), 274 of white ethnicity (72.3%), a median BMI of 25.9 (IQR 22.9–30.1) kg/m2 and 233 (71.5%) had no underlying comorbidities. The median VD3 level of the entire cohort was 55.4 (39.2–68.8) nmol/L. A further breakdown of the participant demographics is presented in table 1.
Aggregated measures within each VD category
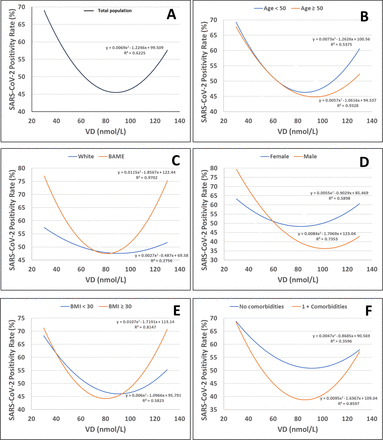
The total population was stratified into four VD categories, 60 (15.8%) with severe deficiency (22.1 (IQR 15.7–26.0) nmol/L), 98 (25.9%) with deficiency (IQR 41.4 (37.3–45.3) nmol/L), 151 (39.8%) with insufficiency (61.0 (IQR 56.3–66.5) nmol/L) and 70 (18.5%) with sufficiency (85.1 (IQR 78.2–95.8) nmol/L) (table 1). The U-curve shown in the total population (figure 1A) shows that below levels of 80 nmol/L, seropositivity increases as the VD level reduces. The trend lines for all the cohort subgroups (figure 1B–F) all broadly follow this U-shaped curve, with plateau points spread between 80 nmol/L and 100 nmol/L.
SARS-CoV-2 seropositivity rate against serum VD levels by (A) Total, (B) Age group, (C) Ethnicity, (D) Sex, (E) BMI and (F) Presence of comorbidities. Seropositivity rate is defined as the number of SARS-CoV-2-positive cases, divided by the total number of cases. The data are represented by a weighted second-order polynomial regression smooth line. The line equation and the R² value are placed beside each corresponding line. BAME, black, Asian and minority ethnic; BMI, body mass index; VD, vitamin D.
VD association with SARS-CoV-2 seropositivity
In the cohort, 208 (54.9%) tested positive for SARS-CoV-2 antispike glycoprotein antibodies (table 2). No significant difference existed between the median VD values of the COVID-19 seropositive (54.2 (34.3–68.6) nmol/L) and COVID-19 negative (57.0 (41.3–68.8) nmol/L) groups (p=0.20) (table 1). Inspection of the total population shows that the COVID-19 seropositive group had 2.75 times the amount of severely deficient participants in comparison to the COVID-19 seronegative group (table 2). When VD was stratified, SARS-CoV-2 seropositivity was 73.3% in the severely deficient group compared with the deficient (45.9%; p=0.001), insufficient (53.0%; p=0.008) and sufficient (56.7%; p=0.049) groups (tables 2 and 3).
The proportion of positive and negative SARS-CoV-2 seroconversions
P value table: comparisons of SARS-CoV-2 seropositivity within subgroups between VD category pairs
Age
Both age subgroups <50 years and ≥50 years displayed similar differences in seropositivity with increasing VD (figure 1B). There was only a significant difference in seropositivity between the severely deficient and insufficient VD levels in the <50 years subgroup (p=0.03) (table 3).
Ethnicity
Change in seropositivity rate with VD level was markedly different between the BAME and white ethnicity subgroups (figure 1C). The BAME subgroup had significantly higher seropositivity rates between severely deficient (78.6%) and insufficient (50.0%) positions; p=0.02 (tables 2 and 3). There was no significant difference in seropositivity rates between severely deficient and insufficient in the white subgroup (p=0.62) (table 3). Both subgroups showed an increase in seropositivity rate with increasing VD from~80 nmol/L to 90 nmol/L, but seropositivity rate increased at a greater rate in the BAME subgroup.
Sex
In both groups of men and women there were significantly lower rates of seropositivity between the severely deficient and deficient graph areas (p=0.006 and p=0.048, respectively) (table 3, figure 1D). Significant difference was found between the severe deficiency category and insufficiency category, and between the severe deficiency category and sufficiency category (p=0.01 and p=0.004, respectively) in the male subgroup (table 3). Seropositivity rate was higher in women in the sufficiency category (61%) (table 2), but this did not reach statistical significance (p=0.13). Men reached a plateau in seropositivity rate at a lower VD level (~80 nmol/L) compared with women (~100 nmol/L) (figure 1D).
Body mass index
With regards to BMI, the change in seropositivity rate with VD level for both subgroups (≥30 kg/m2 and <30 kg/m2) was similar (figure 1E). In both groups the seropositivity rates reduced until VD~80 nmol/L. A significant reduction in seropositivity rate occurred between both the severely deficient and the deficient categories, and between the severely deficient and insufficient categories (p=0.01 and p=0.03, respectively) in the <30 kg/m2 BMI cohort (table 3).
Comorbidities
A significant reduction existed between the severely deficient and deficient areas in the 0 comorbidity group (p=0.005) (table 3). There was also significant difference between the severely deficient and insufficient categories in the 1+ comorbidities subgroup (p=0.045) (table 3). The largest difference in seropositivity rate existed within the insufficient graph area, with 44.1% in the 1+ comorbidities subgroup, and 60.2% in the 0 comorbidity subgroup (table 2), but this did not reach statistical significance (p=0.052).
Groups at risk of severe VD deficiency
VD deficiency is often related to age, sex, BMI, ethnicity and comorbidity so we assessed the impact of these on VD levels and COVID-19 serology seropositivity.
Age
There were no differences in the proportion of the <50 years group in the severely deficient category in comparison to the ≥50 years group (figure 2B, table 1). Within the four VD categories, there was a higher proportion of <50 years individuals represented in all of them (ranging from 62.9% to 78.6%) (table 1). In the severely deficient category, the ratio of ≥50 years:<50 years was 13:47 (ie, 0.28:1) (table 1). The ratio in the sufficient category was larger but did not meet statistical significance (p=0.06), at 0.59:1 (tables 1 and 4). The largest difference existed between the sufficient category and the deficient category (0.27:1) (table 1). This difference reached significance (p=0.04) meaning an individual <50 years is 3.7 times more likely to be VD deficient than VD sufficient (tables 1 and 4).
Proportion of subgroup population by VD category. Each bar represents the proportion (%) of participants that is severely VD deficient (blue) and VD deficient (orange) within the total population (A) and when subgrouped according to age (B), ethnicity (C), sex (D), BMI (E) and comorbidity (F). Comparisons are made between the two subgroups to determine whether there is a significant difference in the proportion of the subgroup within each VD category. BAME, black, Asian and minority ethnic; BMI, body mass index, VD, vitamin D.
P value comparisons between VD category pairs
Ethnicity
Within the severely deficient category, there was a significantly larger proportion of the BAME group (40%) in the category in comparison to the white ethnic subgroup (6.6%); p<0.001 (table 1, figure 2C). The ratio of BAME:white in the severely deficient category (2.3:1) was significantly higher than the BAME:white ratios formed from each of the deficient, insufficient and sufficient VD categories (0.26, 0.21 and 0.32:1, respectively; all p<0.001) (table 4, figure 3C). This indicates that BAME are more likely to be severely VD deficient than VD deficient.
Relative proportions of paired subgroups within VD categories. The proportion (%) of participants who were 1=severely deficient, 2=deficient, 3=insufficient, 4=sufficient within the total population (A). For each of the subgroups of vitamin D status (categories 1–4), the proportion of those differing by age (B), ethnicity (C), sex (D), BMI (E) and comorbidity (F). BAME, black, Asian and minority ethnic; BMI, body mass index; VD, vitamin D.
Sex
There were no differences in the proportion of men compared with women in both the severely VD deficient and VD deficient (figure 2D) groups. There were no significant differences between the proportions of any of the VD comparisons (figure 3D, table 4). The closest to significance (p=0.07) existed between the severely deficient and sufficient groups, with ratios (male:female) of 0.54:1 and 0.25:1, respectively (tables 1 and 4).
Body mass index
There were no statistically significant differences in the proportion of the BMI≥30 kg/m2 subgroup in the severely VDD category versus BMI<30 kg/m2 subgroup (figure 2E). A higher proportion of the BMI<30 kg/m2 subgroup existed within all the VD categories (ranging between 66.3% and 85.7%) (table 1, figure 3E). Significant difference in the proportions was only found between VD categories of deficient and sufficient (p=0.01) (table 4). The ratios of BMI<30 kg/m2:≥30 kg/m2 were 1.94:1 and 6.14:1, respectively (table 1).
Comorbidity status
The proportion of the 0 comorbidity subgroup (which were VDD) was significantly higher (30.5%) compared with the equivalent in the 1+ comorbidities group (18.5%); p=0.046 (figure 2F). In both comorbidity subgroups, the proportions were higher in the deficiency category than in the severe deficiency category. A larger proportion of the 0 comorbidity group existed within each VD category compared with the 1+ comorbidities group (ranging from 55.0% to 72.4%) (table 1, figure 3F). The most equal proportions between the two comorbidity subgroups existed within the insufficiency category (0.82:1) (table 1). A statistically significant difference was found between the insufficiency category and the deficiency category (0.38:1); p=0.007 (table 1). A statistically significant difference was also found between the deficiency category and sufficiency category (p=0.048) (table 4).
Discussion
In summary, statistically significant differences in SARS-CoV-2 seropositivity were found within the entire cohort, and several subgroups across the four VD categories. The subgroups included both sex subgroups; age >50 years subgroup; BAME subgroup; BMI<30 kg/m² subgroup and both comorbidity subgroups. There were no statistically significant differences between the seropositivity rates in the paired subgroups in any VD category. All curves displayed varying U-shaped curves, with ethnicity showing the most variation between subgroups: BAME subjects showed marked increase in seropositivity as VD level moved into the deficient areas, whereas the white cohort showed less variation in seropositivity across the VD continuum.
Seropositivity rate was significantly higher in the severely deficient category in comparison with any of the other VD categories within both the total population and the male subgroup. After a reduction in seropositivity rate between a VD level of 30 nmol/L and ~80 nmol/L, there was an unexpected increase in seropositivity rate beyond VD ~80 nmol/L. This resulted in a U-shaped curve that was reflected within all the subgroups.
A significantly larger proportion of the BAME population were severely VD deficient relative to the total white population. This finding was consistent across the total population, but also both the SARS-CoV-2-positive and SARS-CoV-2-negative BAME groups individually. These results add to the literature that individuals of darker skin are more likely to be VD deficient because of a greater melanin content, reducing the availability of ultraviolet B (UVB) rays for VD3 synthesis.7 This evidence is also consistent with a recent study in the UK which found a significantly higher proportion of VD deficiency among newborns within the BAME ethnic group.13 Regardless of whether VDD is a cause or consequence (or both) of COVID-19, further investigation into VD supplementation in those of BAME ethnic group is warranted.
The U-shaped curves observed in this study challenge the current understanding of the relationship between VD and COVID-19, in which susceptibility is assumed to reduce with increasing VD levels.14 No published studies regarding COVID-19 have reproduced such results, that is, those demonstrating increasing seropositivity at both ends of the VD spectrum. This U-shaped curve, however, has appeared in the wider literature regarding VD. For example, a large sample (n=24 094) study by Amrein et al (2014) investigated the relationship between hospital admission VD and mortality.15 Interestingly, after a reduction in mortality with increasing VD levels, 90-day mortality rate began to increase beyond ~125 nmol/L levels, with an independent predictor of mortality beyond 150 nmol/L. In contrast, a review in 2016 identified all the studies (at the time) that investigated VD level against multiple outcomes within a U-shaped distribution: they concluded that the results were unlikely to be valid due to the lack of consideration of vulnerable individuals taking VD supplementation.16 There has been increased interest during the course of the pandemic of the role of VD supplementation to protect from COVID-19,17 with evidence from meta-analysis that supplementation reduces risk of acute respiratory tract infection.18 However, in our study, only eight individuals were on VD supplementation at the time of enrolment. Only two individuals had VD levels over 80 nmol/L from a total of 51 subjects and therefore would have minimal contribution to the observed U-shaped effect.
The only studies where the U-shaped curves were possibly significant were associated with allergy, due to a changing balance in the Th1/Th2 axis.16 Due to U-shaped curves being identified in several studies against disease risk, further investigation is required to understand this phenomenon. One common explanation is whether the cause of increased disease risk passed a certain VD is due to the individual being on supplementation due to being a clinically vulnerable individual. However, our cohort were healthcare staff with few comorbidities. One possible mechanism of the U-shape curve could be due to induction of fibroblast growth factor-23 at higher levels of 25(OH)D (>100 nmol/L 25(OH)D) and the consequent inhibition of 1-hydroxylase in immune cells.19
There has been interest in the role of VD binding protein (DBP) in relation to COVID-19 infection. This study did not measure DBP; polymorphisms in the DBP gene have been shown to be associated with severity of COVID-19 infection.20 DBP is the main serum binder of 25(OH)D and has an indirect role in the activation of innate immune cells. DBP levels have been shown to drop in patients with ARDS,4 which may indirectly lead to increase in free 25(OH)D which may enhance the availability of 25(OH)D to immune cells. Furthermore, single nucleotide polymorphisms contribute a relatively small proportion of overall VD availability.21 Therefore, the role of DBP in the setting of COVID-19 remains unclear.
Limitations
There are a number of limitations to this study. The aggregation of multiple ethnicities into a singular ‘BAME’ subgroup provided a challenge, and due to the study population did not allow further subcategorisation of ethnicity for which there may be further susceptibilities.6 The study was conducted during the first wave of the COVID-19 pandemic and therefore predated mass testing for COVID-19 and PCR testing was only being conducted within hospitalised patients. Individuals were recruited based on displayed symptoms of COVID-19 and isolation. A limitation is that we have looked at seropositivity, rather than infection, though the used assay has a very high sensitivity for PCR proven disease. Other members of staff isolating for symptoms suggestive of COVID-19 who were not antibody positive may have had other respiratory tract infection or alternative diagnosis. Inclusion bias is another limitation raised consistently; individuals were recruited based on displayed symptoms of COVID-19 and isolation. This potentially increased the risk of selecting individuals that were more susceptible to COVID-19, regardless of their VD levels. Furthermore, healthcare workers had a higher risk of COVID-19 infection, particularly those who were patient facing, and so the population is not easily generalisable outside of a healthcare population. The amount of time the subjects may have been infected was not considered, and so VD levels versus severity of disease (alongside confounding influences) will affect the ability to interpret the results. As the median duration from symptom onset to testing was 48 days, this would have allowed sufficient time to develop an antibody response following infection. Another issue which is raised consistently in similar literature is seasonal variability in VD;22 however the participants were recruited within a tight timeframe in May, which should reduce effect of seasonal variation. There could be other confounding factors such as nutritional intake and use of fortified foods which were not assessed in the study and are another limitation. The U-shaped curves were derived by grouping the samples into four VD bins, principally because there were limited numbers at each end of the VD spectrum.
Conclusions
Our study has shown a U-shaped relationship for COVID-19 seropositivity in UK healthcare workers. Further investigation is required to determine whether high VD levels can have a detrimental effect on COVID-19 susceptibility. Future randomised clinical trials of VD supplementation could potentially identify ‘optimal’ VD levels. This would allow for targeted therapeutic treatment for at-risk groups such as those within the BAME ethnic group.
Data availability statement
Data are available upon reasonable request. The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the London-Camden and Kings Cross Research Ethics Committee (20/HRA/1817). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors thank the staff of University Hospitals Birmingham NHS Foundation Trust who kindly volunteered for this study. The authors also thank the research staff of the Birmingham Wellcome NIHR Clinical Research Facility who undertook the staff facing assessments. The authors also thank colleagues at the Clinical Immunology Service for overseeing recruitment and sample processing.
References
Footnotes
STL and WRM are joint first authors.
AS and DRT are joint last authors.
Contributors DRT is the guarantor of the data, accepts full responsibility for the work and the conduct of the study, has access to the data, and controlled the decision to publish.DRT and AS conceptualised the study. AAF, SEF, CW, JED, AS, AGR and DRT contributed to data acquisition. WRM, STL and AS analysed the data. All authors contributed to data interpretation. STL, WRM, DP, AS and DRT drafted the manuscript. All authors contributed to the review and approval of the final copy of the manuscript. DRT is the guarantor of the study.
Funding Research support was provided by the National Institute for Health Research (NIHR)/Wellcome Trust Birmingham Clinical Research Facility. Laboratory work was done at the Clinical Immunology Service of the University of Birmingham and the Biochemistry department within the University Hospitals Birmingham NHS Foundation Trust.
Competing interests MH reports personal fees from Thornton Ross, outside the submitted work.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.