Article Text
Abstract
Introduction Updated treatment guidelines for acute hypercapnic respiratory failure (AHRF) in chronic obstructive pulmonary disease (COPD) with non-invasive ventilation (NIV) in 2016 recommended a rapid increase in inspiratory positive airway pressure (IPAP) to 20 cm H2O with possible further increase for patients not responding. Previous guidelines from 2006 suggested a more conservative algorithm and maximum IPAP of 20 cm H2O.
Aim To determine whether updated guidelines recommending higher IPAP during NIV were related with improved outcome in patients with COPD admitted with AHRF, compared with NIV with lower IPAP.
Methods A retrospective cohort study comparing patients with COPD admitted with AHRF requiring NIV in 2012–2013 and 2017–2018.
Results 101 patients were included in the 2012–2013 cohort with low IPAP regime and 80 patients in the 2017–2018 cohort with high IPAP regime. Baseline characteristics, including age, forced expiratory volume in 1 s (FEV1), pH and PaCO2 at initiation of NIV, were comparable. Median IPAP in the 2012–2013 cohort was 12 cm H2O (IQR 10–14) and 20 cm H2O (IQR 18-24) in the 2017–2018 cohort (p<0.001). In-hospital mortality was 40.5% in the 2012–2013 cohort and 13.8% in the 2017–2018 cohort (p<0.001). The 30-days and 1-year mortality were significantly lower in the 2017–2018 cohort. With a Cox model 1 year survival analysis, adjusted for age, sex, FEV1 and pH at NIV initiation, the HR was 0.45 (95% CI 0.27 to 0.74, p=0.002).
Conclusion Short-term and long-term survival rates were substantially higher in the cohort treated with higher IPAP. Our data support the current strategy of rapid increase and higher pressure.
- Non invasive ventilation
- COPD Exacerbations
Data availability statement
Data may be obtained from a third party and are not publicly available. No additional data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Treatment of acute hypercapnic respiratory failure (AHRF) due to acute exacerbation of chronic obstructive pulmonary disease (COPD) with non-invasive ventilation (NIV) is well documented and widely implemented. With further attention to efficiency of treatment, updated Danish national NIV guidelines in 2016 recommended a paradigm shift with a new algorithm of initial rapid rise in pressure and use of higher pressures.
WHAT THIS STUDY ADDS
We performed a retrospective cohort study comparing patients with COPD with AHRF requiring NIV in 2012–2013, before introduction of updated guidelines, and in 2017–2018. Short-term and long-term survival rates were higher in patients undergoing NIV in 2017–2018 compared to those in 2012–2013.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
It seems plausible that improved survival is an effect of treatment with higher pressures and our data support the current strategy.
Introduction
For patients with chronic obstructive pulmonary disease (COPD), acute hypercapnic respiratory failure (AHRF) secondary to acute exacerbation of COPD (AECOPD) is associated with substantial mortality and risk of intubation. Treatment of AHRF with non-invasive ventilation (NIV) has improved these outcomes since the treatment was introduced in 1990.1 2 Even though early studies showed more efficient resolution of respiratory acidaemia when using a inspiratory positive airway pressure (IPAP) of 20 cm H2O than 12 cm H2O,1 early NIV treatment guidelines recommended quite conservative pressure settings. The Danish Society of Respiratory Medicine suggested initial IPAP of 12 cm H2O, to be increased to a maximum 20 cm H2O3 but in a Danish retrospective study of 286 patients undergoing in-hospital NIV treatment in 2012–2013 median maximum IPAP was only 12 cm H2O (IQR 12–14), that is, suggesting that the recommended use of higher IPAP was not implemented fully.4 With evidence from randomised controlled trials (RCTs) on home long-term NIV (LT NIV) treatment of patients with COPD the use of higher IPAP to reduce PaCO2 has become more common.5 Updated Danish treatment guidelines for AHRF in 2016 recommend initial IPAP of 12–15 cm H2O and a rapid increase to 20 cm H2O within the first 30 min of treatment and possible further increase if the patient is not responding.6 Recommendations were similar to the 2016 British Thoracic Society and Intensive Care Society ‘Guideline for the ventilatory management of AHRF in adults’.7 The efficacy and safety of using higher IPAP are mainly derived from data on out-of-hospital patients with COPD in LTNIV treatment and the high-intensity NIV concept.8 9 Less is known of the short-term and long-term effects of increased IPAP in treatment of AHRF. Further RCT, comparing NIV with low and high IPAP settings, does not seem to be likely nor ethically justified.
The aim of this study was to determine whether NIV treatment according to updated guidelines, recommending rapid increase and higher IPAP, compared with earlier treatment regimens using lower IPAP, was associated with better outcome in patients with COPD admitted with AHRF.
Materials and methods
Study design
In this retrospective cohort study of patients admitted with AHRF due to AECOPD requiring acute NIV, we compared patients treated according to guidelines from 2006 recommending quite conservative settings and lower IPAP (‘2012–2013 cohort’), with patients treated according to guidelines from 2016 recommending higher IPAP (‘2017–2018 cohort’). We included all patients who received NIV due to AECOPD with AHRF at the Respiratory Unit, Department of Internal Medicine, Gentofte Hospital (Capital Region, Denmark) from 1 January 2012 to 31 December 2013, and from 1 January 2017 to 31 December 2018. Patients with AHRF due to other causes than AECOPD were not included.
Study population
All patients admitted due to AECOPD and/or respiratory failure were screened. Patients who underwent NIV treatment for AHRF in the respiratory ward were included. Patients who had NIV initiated at the intensive care unit (ICU) and were later transferred to the respiratory ward, still requiring NIV, were also included. The COPD diagnosis was defined in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) definition10 and assessed by the treating physician based on clinical history, physical examination and spirometry. Initial examination including arterial blood gas analysis, blood samples and chest X-ray were performed at admission in the acute medical department. Acute medical treatment of AECOPD included bronchodilators, systemic corticosteroids, controlled oxygen therapy and, if indicated, antibiotics. Indication for NIV was pH <7.35 and PaCO2 >6 kPa after 1–2 hours of initial treatment, in accordance with GOLD.10 Acute medical treatment of AECOPD, oxygen therapy recommendations and indication for NIV treatment were unchanged during the study period.
Data collection
Screening and data collection were performed according to a predefined protocol including baseline characteristics at index admission, data concerning index admission, and a 1-year follow-up of events, number of readmissions and death. Data on patients undergoing treatment in 2012–2013 cohort were already available, collected in 2014 and has previously been used in two publications.4 11 Patients in the 2017–2018 cohort were screened for inclusion or exclusion in accordance with the protocol for the 2012–2013 cohort. All data, including data regarding mortality, were collected from electronic patient medical records, which correspond and are automatically updated through the Danish Cause of Death Registry.
NIV treatment
NIV treatment guidelines for AHRF from 2006 recommended an initial IPAP of 12 cm H2O and with a maximum increase of IPAP to 20 cm H2O. The 2016 NIV treatment guidelines for AHRF recommended a rapid increase in IPAP from initial IPAP of 12 to 15, to IPAP of 20 cm H2O within the first 30 min after initiating NIV and that further increase could be considered if necessary, to acquire normalisation of pH and a decline in PaCO2. In 2017–2018, these recommendations were the general clinical practice at the department. Treatment with NIV was performed in the respiratory ward with specially trained nursing staff, according to a standardised algorithm for collection of blood gas sample and adjustment of NIV setting. The ventilator settings used were pressure-targeted spontaneous/timed mode. NIV was delivered semicontinuous during the first 24 hours. As the patient’s condition improved, and pH and PaCO2 were normalised, a plan for NIV weaning was made. For successful withdrawal of treatment, the time with NIV was gradually reduced during the day while continuing overnight NIV. NIV failure was defined as patients not successfully responding to NIV, either requiring endotracheal intubation and ICU treatment or death for patients with NIV as ceiling of treatment. ‘Do not intubate/Do not resuscitate’ (DNI/DNR) orders were either classified as ‘initial order’, placed within 2 hours of the admission, or ‘late order’, placed later than 2 hours of admission during NIV treatment.
Statistical analysis
Primary outcome was in-hospital mortality. Secondary outcomes were 30-day and 1-year mortality and adherence to pressure setting according to treatment guidelines.
Categorical variables are presented as numbers and percentages and analysed using Pearson’s χ2 test. Continuous variables are presented as medians and IQRs and analysed using Mann-Whitney U test.
Survival analysis for in-hospital mortality, 30-day mortality and 1-year mortality was conducted. The cumulative probability of survival is presented as a Kaplan-Meier survival plot, compared using log-rank test. To control for potential confounders, logistic regression and a Cox proportional hazards regression analysis were performed, adjusted for age, sex, forced expiratory volume in first second (FEV1) and pH at initiation of NIV, using a complete case analysis. Results are presented as odds ratio (OR) hazard ratio (HR) with a 95% confidence interval (95% CI). Furthermore, to assess robustness, calculation of E-values for estimation of the required strength of potential unmeasured confounders was performed.
Statistical analyses were performed in RStudio V.1.2.5001.
Patient and public involvement
Patients or public were not involved in the design of the study.
Results
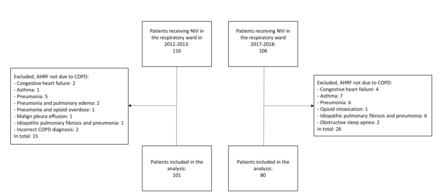
181 patients were included in the analyses: 101 patients in the 2012–2013 cohort with low IPAP regime and 80 patients in the 2017–2018 cohort with high IPAP regime (figure 1).
Flowcharts of the cohorts in the 2012–2013 and 2017–2018.
AHRF, acute hypercapnic respiratory failure; NIV, non-invasive ventilation, COPD, chronic obstructive pulmonary disease.
Baseline characteristics and data from index admission are presented in table 1. Overall, the two cohorts were similar on baseline chronic variables and admission data from the acute index admission. There was, however, a significant difference in pack-years; the 2012–2013 cohort had a history of a median of 40 pack-years (IQR 30–50) compared with 50 (IQR 40–60) in the 2017–2018 cohort. There was a trend towards higher body mass index in the 2017–2018 cohort, but not statistically significant. Respiratory rate on initiation of NIV was higher in the 2012–2013 cohort, 28 breaths per minute (IQR 24–32) compared with 24 breaths per minute (IQR 20–28) in the 2017–2018 cohort (p=0.001). Median IPAP in the 2012–2013 cohort was 12 cm H2O (IQR 10–14) compared with 20 cm H2O (IQR 18–24) in the 2017–2018 cohort (p<0.001). Spirometry measurements and FEV1 were missing in 28 patients, as they had been diagnosed and received outpatient care at clinics outside the hospital.
Baseline characteristics and index admission data
In the 2012–2013 cohort 47 patients (46.5 %) had an initial DNI/DNR order, and 14 patients (13.9 %) had a late order. In the 2017–2018 cohort, 24 patients (30.8 %) had an initial DNI/DNR order and 9 patients (11.5 %) had a late order. NIV failure and the need for intubation and ICU treatment occurred in eight patients (8%) in the 2012–2013 cohort, and two patients (2.5%) required intubation (p=0.123).
Mortality and survival
Data on mortality are presented in table 2. In-hospital mortality was 40.5% in the 2012–2013 cohort to 13.8% in the 2017–2018 cohort (p<0.001). Furthermore, 30-day mortality and 1-year mortality were also significantly lower in the 2017–2018 cohort.
Mortality data
Unadjusted 1-year survival analysis with Kaplan-Meier plot is presented in figure 2. With the log rank test the cumulative estimate of survival was significantly higher in the 2017–2018 cohort (p=0.002). The adjusted regression model and Cox proportional hazards model are presented in table 3. The models are adjusted for age, sex, FEV1 and pH at NIV initiation, and 153 patients were included in the complete case analysis. Patients in the 2017–2018 cohort had higher 1-year survival rates both with unadjusted (HR 0.50, 95% CI 0.32 to 0.77, p=0.002) and adjusted Cox proportional hazards model (HR 0.45, 95% CI 0.27 to 0.74, p=0.002). E values for the adjusted ORs and HRs are reported in table 3. All E values were 2.8 or larger, indicating that rather substantial unmeasured confounding, beyond the measured covariates, would be required to dismiss the effect estimate.
Survival analysis
Kaplan-Meier survival plot, survival 365 days after initiation of non-invasive ventilation for treatment of acute hypercapnic respiratory failure due to COPD.
Discussion
In this single-centre retrospective study, comparing two cohorts, treated for AHRF with NIV in 2012–2013 and 2017–2018, before and after introduction of a new treatment guideline in 2016 recommending a more rapid rise in pressure and use of higher IPAP, we found a significantly better short-time and long-time survival in the later cohort. To our knowledge, this is the first study to show a lower mortality when providing NIV with higher IPAP i for treatment of AHRF secondary to AECOPD. Additionally, data from the present study suggest that the risk of mortality following hospitalisation is highest immediately after discharge. For patients who survived the first 30 days post hospital discharge, the 1-year survival remained high (figure 2, Kaplan-Meier plot). This is in accordance with studies on survival after hospitalisation with AECOPD.12
Our data show that the new treatment guidelines were well implemented, with a median IPAP of 20 cm H2O in 2017–2018 compared with 12 cm H2O in 2012–2013. Apart from updated recommendations regarding the algorithm for pressure settings for NIV, standard treatment of AECOPD and the indication for NIV treatment were unchanged from 2012 to 2018.
Over the recent years, attention to improve suboptimal NIV treatment has risen. Insufficient pressure settings, especially inadequate IPAP, will not increase alveolar ventilation significantly. Focus on a more rapid improvement of pH within the first 4 hours after initiation of NIV is essential to reduce relative risk of NIV failure.13 The improved survival in patients treated with higher IPAP in the present study, with otherwise comparable cohorts, suggests that using higher IPAP is more efficient to resolve AHRF and thus acute respiratory acidaemia. A rapid improvement of ventilation and patient’s recovery may also increase patient acceptance of NIV treatment and reduce the risk of NIV failure. Furthermore, shortening length of treatment reduces risks of complications associated with hospitalisation and immobilisation; pulmonary embolism, hospital acquired pneumonia, and loss of physical mobility.
Other aspects in general COPD care changed from 2012 to 2018 and may have contributed to preventing admissions with AHRF and improving patient survival. Treatment with LT NIV has been shown to prevent hospitalisation in the subgroup of most severely ill patients with COPD.5 LT NIV was introduced at the current department in the year 2000 and has become more frequent for the small subgroup of patients with COPD with chronic stable hypercapnia as well as for selected patients with recurrent exacerbations with AHRF.14 Pharmaceutical treatment with long-term prophylactic azithromycin, as anti-inflammatory therapy, to reduce risk of exacerbations in patients with a history of recurrent exacerbations has also become more widespread.15
In research regarding treatment survival, mortality rates in observational studies are commonly higher compared with RCTs. Risk of in-hospital mortality was 99 per 1000 (95% CI 70 to 139) in the 2017 Cochrane meta-analysis analysing the results of 12 RCTs.2 Published cohort studies on survival of AHRF show great variation in mortality. In a Danish nationwide register-based study of 12 847 patients admitted for AECOPD and treated with NIV in 2004 to 2011, median age 73.7 (IQR 66–80) years, in-hospital mortality was 24.4% (95% CI 24.3 to 24.5).16 Among patients alive at discharge, 26.4% (95% CI 26.3 to 26.5) died within 1 year. In a smaller Dutch retrospective single-centre study of 78 patients admitted with AHRF requiring NIV in 2009 to 2011, in-hospital mortality was 14.1%, with 1-year mortality of 43.6%.17 Mean age of the cohort was 71.0 (SD ±10.7) years, median FEV1 39.0% (IQR 28.6–52.9) and median PaCO2 before NIV 10.0 (IQR 8.5–11.2) kPa. Data from the national COPD audit in the UK in 2008 and 2014 showed a significantly improved in-hospital mortality of 24.9% and 16.8%, respectively.18 Findings were explained due to better adherence to guideline recommendations of ventilatory settings, although data on pressure settings were not recorded, as well as improvements of general care such as controlled oxygen and early antibiotic treatment. Further retrospective data from the UK compared two cohorts, 2004–2010 and 2013–2017, of patients with COPD and first episode of ward‐based NIV.19 In-hospital mortality rates were 17.6% and 20.5% (p=0.378). Characteristics of the two cohorts were as follows, respectively: median age of 72.1 (IQR 64.2–78.8) and 69.9 (IQR 63.7–76.9) years, FEV1 of 32 (IQR 24–39) and 35 (IQR 27–47)%, PaCO2 before NIV of 9.36 (IQR 27–47) and 10.34 (IQR 8.89–11.8) kPa and maximum IPAP of 15 cm H2O (IQR 12–18) and 18 (IQR 15–21).
Limitations of the present study are the limitations associated with a retrospective cohort study, the risk of selection and information bias with imprecise outcome registration and uneven collection of data. A potential selection bias is different selection of patients to whom physicians offered NIV treatment to in the two cohorts. As NIV was a newer, advanced technology in 2012–2013, physicians could have been more prone to select the most severely ill patients for NIV as rescue treatment, whereas in the later cohort, NIV could have been offered more broadly to patients with AHRF, despite the severity of acidaemia, comorbidities and performance status. However, baseline characteristics and blood gas levels of pH and PaCO2 at initiation of NIV were comparable in the cohorts, which contradicts a selection bias. As a single-centre study with a small size cohort, there is the potential risk of overstating positive results and the lack of external validity required to support a more widespread generalisation of the results. The in-hospital mortality was notably high in both cohorts compared with the previously mentioned studies, reflecting the demographic composition of patients treated at the hospital, with median age of 78 in both cohorts. Other factors associated with death such as FEV1 and pH and PaCO2 before NIV are in accordance with data from previous studies. NIV was ceiling therapy for 60% of patients in the 2012–2013 cohort and 42% of patients in the 2017–2018 cohort. This is also reflected in the small percentage of patients transferred to the ICU due to NIV failure. Furthermore, about one out of four DNI/DNR orders were placed later than 2 hours of admission in both cohorts.
Conclusion
This observational study shows that patients with AHRF due to AECOPD treated with NIV using higher IPAP in 2017–2018, according to contemporary guidelines, had significantly better short-term and long-term survival compared with patients treated with lower IPAP in 2012–2013. It seems plausible that improved survival is an effect of higher IPAP; however, further research is needed, preferably multicentre studies including a larger number of patients.
Data availability statement
Data may be obtained from a third party and are not publicly available. No additional data are available.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by our local ethics committee (R-20067943) and data monitoring board (P-2020-881).The study is retrospective and in accordance with the approval from the ethics committee. Consent for participation was not required.
References
Footnotes
Contributors CH, PMN, CPH and JTW designed the study. PMN, DBR and TPS collected data. Statistical analysis was performed by CH, PMN and NH. CH prepared and drafted manuscript and completed submission. CH and JTW are responsible for the overall content as the guarantor and accept full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish. All authors contributed revision and final approval of manuscript.
Funding This study was supported by the local research council at Herlev and Gentofte Hospital.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.